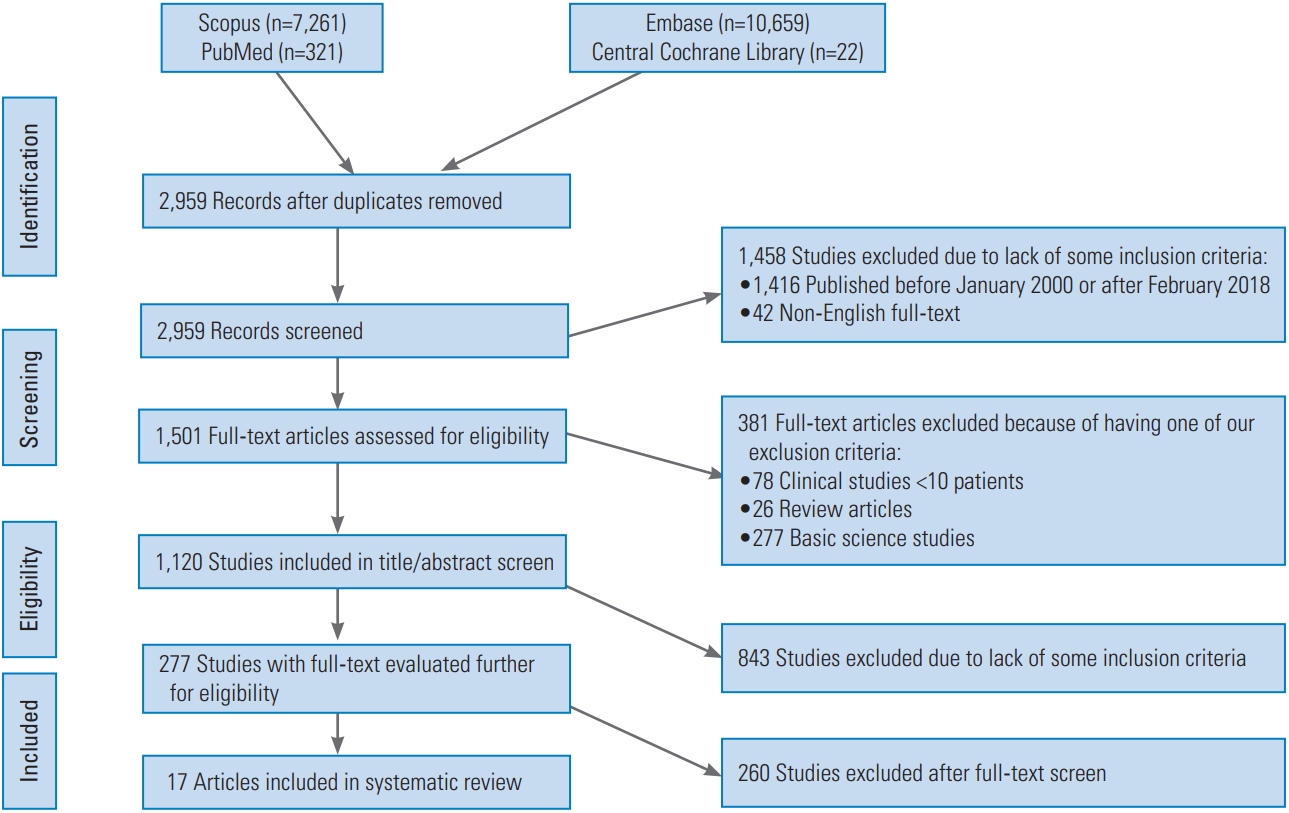
|
 |
- Search
| Asian Spine J > Volume 13(6); 2019 > Article |
|
Abstract
Notes
Author Contributions
AB formulated the research idea and main objectives. AB and CK started the project by collection of data and reviewing the literature. AB and SB designed the study and determined the inclusion and exclusion criteria. CK drafted the tables and created the PRISMA figure under the supervision of AB. OI and AB drafted the main bulk of the manuscript. SB and ZT critically reviewed the manuscript and participated in writing and editing the final draft. ZT is the senior supervisor of the project at all steps. AB and OI interpreted the collected data and reached the conclusion. AB, OI, CK, SB, and ZT approved the final project and held responsible for the entire project under the leadership of AB as a first and corresponding author.
Table 1.
| Author (year) | Drug(s) examined | Objective | Relevant result/conclusion | Type of study | Level of evidence | |
|---|---|---|---|---|---|---|
| Farrokhi et al. [16] (August 2016) | Methylene blue | To examine the effects of MB on postoperative pain and QOL after posterior pedicle screw fixation for thoracolumbar fractures, | - | Significantly lower VAS in the MB group at 2 months (p <0.001) and 6 months (p =0.028). | HCT | Level 1 |
| - | Significantly lower mean ODI score in the MB group at 2 months (p =0.001) and at 6 months (p =0.016). | |||||
| Farrokhi et al. [13] (January 2016) | Methylene blue | To examine the effects of MB on postoperative LBP and QOL after lumbar open discectomy. | - | Significantly lower VAS scores for LBP in the MB group 24 hours (p <0.001) and 3 months (p =0.019). | RCT | Level 1 |
| - | No significant difference on radicular pain scores in both MB and placebo groups (p=0.64). | |||||
| - | Greater reduction in LBP in the MB group (p =0.023). | |||||
| - | Significantly improved functional QOL postoperative 3 months in both groups (p <0.001). | |||||
| Konno et al. [11] (May 2016) | Duloxetine (SNHi) | To evaluate the effectiveness and tolerability of duloxetine monotherapy in Japanese patients with CLBP. | - | Duloxetine monotherapy reduced the BPI score of 2 points at week 14 (p =0.0010). | HCT | Level 1 |
| - | No meaningful changes in vital signs, laboratory studies and heart functions are observed with Duloxetine use. | |||||
| Fann et al. [12] (January 2015) | VenlafaxineXR (SNRI) | To evaluate the benefits and tolerability of Venlafaxine XR use in patients suffering from MDD or dysthymic disorder due to chronic SCI. | - | Improvement on the Maier subscale group (p =0.02). | RCT | Level 1 |
| - | No meaningful improvement on HAM-D17-item version (p =0.42). | |||||
| - | Less SCI-related disability than placebo on Sheehan Disability Scale (p =0.005). | |||||
| - | Venlafaxine XR was well tolerated and effective antidepressant in SCI related disabilities and depression. | |||||
| Richards et al, [4] (April 2015) | Venlafaxine XR (SNHi) | To evaluate the efficacy of Venlafaxine XR in SCI pain and in MDD to show its viability on both neuropathic and nociceptive pain. | Similar HAM-D and GAD-7 scores as placebo on neuropathic pain (p =0.815); but statistically and clinically meaningful reductions in nociceptive pain intensity score in Venlafaxine XR group (p =0.001). | HCT | Level 1 | |
| Thomson et al. [15] (January 2009) | Antidepressants | To compare the demographics of patients with FBSS to ones with other painful conditions in the PROCESS trial. | 38% of patients with FBSS were found on antidepressants. | RCT | Level 1 | |
| Kumar et al. [14] (October 2005) | ||||||
| Cardenas et al. [10] (November 2002) | Amitriptyline (TCA) | To investigate the efficacy of Amitriptyline on chronic pain alleviation and pain-related dysfunction improvement in patients with SCI. | - | No significant change on pain intensity or pain-related disability is observed. (API, SF-MPQ, BPI Pain Interference, CES-Dr, FIM, SWLS, and CHART scales). | HCT | Level 1 |
| - | Findings do not support Amitriptyline use for chronic pain treatment. | |||||
MB, methylene blue; QOL, quality of life; VAS, Visual Analog Scale pain score; ODI, Oswestry Disability Index; RCT, randomized controlled trial; LBP, lower back pain; SNRI, serotonin and norepinephrine reuptake inhibitors; CLBP, chronic low back pain; BPI, Brief Pain Inventory; XR, extended release; MDD, major depressive disorder; SCI, spinal cord injury; HAM-D, the Hamilton Depression Rating Scale; GAD-7, General Anxiety Disorder Scale-7; PROCESS, Prospective Randomized Controlled Multicenter Trial of the Effectiveness of Spinal Cord Stimulation; FBSS, failed back surgery syndrome; TCA, tricyclic antidepressant; API, average pain intensity; SF-MPQ, Short Form McGill Pain Questionnaire; CES-Dr, Center for Epidemiologic Studies-Depression Scale; FIM, Functional Independence Measure; SWLS, Satisfaction With Life Scale; CHART, Craig Handicap Assessment and Reporting Techniques.
Table 2.
| Author (year) | Drug(s) examined | Objective | Relevant result/conclusion | Type of study | Level of evidence | |
|---|---|---|---|---|---|---|
| Saraykar et al. [21] (2018) | SSRI | To measure the association of SSRI with decreased BMD in elderly women >65 years old. | - | No significant difference between SSRI users and nonusers in the BMDs at the lumbar spine (p =0.275). | CSS | Level 4 |
| - | Similar T-scores in two groups at the spine level (p =0.393). | |||||
| Elsamadicy et al. [7] (Apr 2017) | SSRI, SNRIs, others | To determine whether depression or receiving antidepressants can be independent risk factors for postoperative delirium in spine deformity patients after spinal surgery | - | Depression is an independent predictor of postoperative delirium after spine surgery in spinal deformity patients (p =0.01). | RCS | Level 3 |
| - | No difference between antidepressants users and nonusers in postoperative delirium (p =0.56). | |||||
| Schadler et al. [8] (Feb 2017) | SSRI | To detect the association of blood loss and transfusion with one-level spine surgery | SSRI intake is a predictor for: blood loss (34% increase, p =0.015); blood transfusion (OR, 4.55; p =0.029). | CCS | Level 3 | |
| Sayadipour et al. [20] (Apr 2016) | Antidepressants | To detect the economic effects of antidepressants usage on elective spinal surgery. | 36% increase in total charges; 22% increase in cost; 19% increase in fixed cost; but none of the above was statistically significant. | CCS | Level 3 | |
| Robertson et al. [19] (2016) | Amitriptyline (GBP+TCA) | Evaluating benefits and adverse effects of GBP inclusion to AMP treatment for chronic sciatica | GBP inclusion to AMP mono-treatment has showed increased efficacy in terms of VAS reduction (p <0.0001) and ODI reduction (p =0.008) scores. | PCS | Level 2 | |
| Reduced efficacy measured in patients having adverse effects (VAS, p=0.08; ODI, p =0.01). | ||||||
| Sajan et al. [22] (Mar 2016) | SSRI | To examine the relationship between SSRI intake and perioperative blood product transfusion in surgical patients with high risk of perioperative bleeding including spinal fusion surgery. | - | Increased risk of exposure to allogeneic hemostatic blood products (OR, 2.2; CI, 1.2–3.98). | PCS | Level 2 |
| - | No effect modification was found between SSRI use and surgery type, age, sex, or concurrent antiplatelet therapy. | |||||
| Elsamadicy et al. [6] (2016) | Antidepressants (not specified) | To detect the association between treating depression before cervical spine surgery and patient satisfaction. | No difference at postoperative years (1 and 2) between antidepressants users and nonusers in terms of neck pain VAS, NDI, SF-12 PCS, and SF-12 mental component score. | RCS | Level 3 | |
| Rauma et al. [17] (Apr 2015) | SSRI, SNRI, TCA | To detect the association of major depressive disorder and antidepressants use with bone mineral density in men. | Recurrent MDD was associated with lower spinal BMD (-4%) | CSS | Level 4 | |
| Sayadipour et al. [9] (Jan 2012) | SSRI, SNRI, TCA | To determine if antidepressants usage can be associated with increased bleeding at elective spinal surgery, | - | Patients on antidepressants: 23% more blood loss (p=0.01); 2.5 times greater loss than the matched control group measured in anterior/posterior lumbar fusions operations; 33.3% longer hospital stay (1 day more, p =0.0001); SSRI/SNRI showed higher blood loss (832 mL increase, p =0.01) | CCS | Level 3 |
| - | PLF group: higher blood loss (p =0.032); higher blood loss in patients taking Bupropion (708 mL, p =0.023); longer hospital stay (0.8 days more, p =0.0004) | |||||
| Walid etal. [18] (2010) | Antidepressants (not specified) | To measure the prevalence and impact of mood altering medications and opioids in patients undergoing spine surgery on hospital cost. | 25.4% of spine surgery patients were on antidepressants showing (p <0.05) in both: longer hospital stay; higher hospital cost | CSS | Level 4 | |
SSRI, selective serotonin reuptake inhibitors; BMD, bone mineral density; CSS, cross sectional study; SNRI, serotonin and norepinephrine reuptake inhibitors; RCS, retrospective cohort study; OR, odds ratio; CCS, case control study; GBP, gabapentin; TCA, tricyclic antidepressant; AMP, amitriptyline; VAS, Visual Analog Scale pain score; ODI, Oswestry Disability Index; PCS, prospective cohort study; CI, confidence interval; NDI, Neck Disability Index; SF-12, Short Form-12; PCS, Physical Component Score; MDD, major depressive disorder; PLF, posterior lumbar fusion.