Electron Microprobe Analysis and Tissue Reaction around Titanium Alloy Spinal Implants
Article information
Abstract
Study Design
A retrospective study of tissue surrounding titanium alloy spinal implants was performed using histological and electron microprobe analysis.
Purpose
To identify the metal debris generated by spinal implants, and then to evaluate the electron microprobe analysis results and the histological response of soft tissue surrounding the spinal implants.
Overview of Literature
Microscopic metal particles from the soft tissue surrounding joint arthroplasty have been shown to activate a macrophage response that leads to bone resorption and increased inflammation. The effect of unintended wear particles in spinal instrumentation remains a clinical concern.
Methods
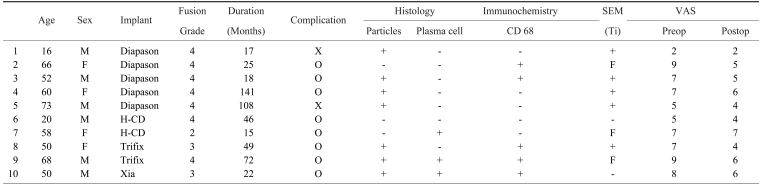
Ten patients (average age, 51.3 years), 6 men and 4 women, who had undergone previous lumbar fusions using pedicle screw instrumentation and who were now undergoing revision surgery were included in the study. The tissues obtained from the adjacent area of these implants were analyzed by light microscopy, immunohistochemistry and scanning electron microscope. After the removing the spinal implants, the changes of back pain and the spinal fusion were assessed.
Results
There were metal particles in the soft tissue in 7 cases. Histological finding observed mild chronic inflammation surrounding the deposition of the metal particles and the anti Cotrel-Dubousset 68 positive macrophages were observed at tissue adjacent to the metal particles in 5 patients. Scanning electron microscopy of the specimens showed metallic debris within the tissue and mapping of the metallic particles revealed the distribution of titanium in the tissue in 5 cases. Nine patients had successful relief of back pain after removing the spinal implants. Improvement of the back pain may be an association macrophage response rather than the metal particle.
Conclusions
The presence of metallic particles generated from spinal implants may serve as the impetus for a late-onset inflammatory response and late operative site pain.
Introduction
The clinical outcomes of the side effects caused by metal debris generated from the micromotion of orthopedic implants have been reported1-4, and the use of metal instrumentation, particularly titanium, for spinal fusion has introduced the possibility of generating microscopic metal particles that may be deposited in either the paraspinal soft tissues or on the neural elements5-11.
Dubousset et al.7 reported late complications in 18 patients managed with posterior stainless steel Cotrel-Dubousset (CD) instrumentation with an average follow-up of 34 months after instrumentation. Histopathology revealed acute and chronic inflammation with granuloma formation at the instrumentation transverse connector site, which necessitated the removal of the instrumentation. The etiology of this complication was considered to be a sterile inflammatory reaction secondary to fretting corrosion of the instrumentation. Wang et al.12 reported metal debris associated with the use of titanium implants in nine patients. It was observed that wear debris is generated by the use of titanium spinal instrumentation in patients with pseudarthrosis, and these particles activate a similar macrophage cellular response in the spinal tissues to that observed in the surrounding joint prostheses. Kasai et al.13 reported that approximately one third of patients with titanium alloy spinal implants exhibited abnormal serum or hair metal concentrations at a mean time of 5.1 years after surgery and that titanium or aluminum may travel to distant organs after dissolution of metals from the spinal implants.
Microscopic metal particles from the soft tissue surrounding joint arthroplasty have been shown to activate a macrophage response that leads to bone resorption and increased inflammation. The use of titanium spinal implants for spine surgery can result in the generation of wear debris in the spine. The toxic effects of these metal particles are also of great concern because the neural elements are widely exposed during spinal decompressions. The purpose of this study was to identify metal debris generated by titanium alloy spinal instrumentation, and then to evaluate the electron microprobe analysis results and the histological response of soft tissue surrounding the spinal implants.
Materials and Methods
This study included 10 patients, 6 men and 4 women, (average age, 51.3 years), who underwent revision surgery from January 2000 to May 2005 at the Kwangju Christian Hospital after having undergone a previous lumbar decompression and fusion with titanium pedicle screw instrumentation, including 6 Diapason instrumentation cases, 3 Trifix instrumentation cases, and 1 Xia instrumentation case. The reason for the revision surgery was hardware removal for back pain without any specific cause in 2 cases and abnormal radiological findings in 8 cases, including periosteal reaction surrounding the pedicle screw in 5 cases, screw breakage in 3 cases, and 1 case of rod failure (Table 1). None of these patients had been identified with draining sinuses or clinical and/or laboratory signs of infection at any time after implantation.
Soft tissue samples were obtained for light microscopic and scanning electron microscopic evaluation. At the time of surgery, soft tissue samples were taken from the dark brown or black discolored area macroscopically surrounding the deposition of metal particles released in the soft tissues (7 cases, Fig. 1A) and from the pseudomembrane in the vicinity of the connection area of the metal rods and screws in 3 cases. All the specimens were cultured for the possibility of a subclinical infection. For the light microscopy evaluation, all the specimens were fixed in 10% buffered formalin, and processed using routine light microscope techniques (embedded in paraffin, cut in 5 µm slices, and stained with hematoxylin-eosin). The specimens were also examined for debris-induced effects on the soft tissue surrounding the deposition of the metal particles. Under a light microscope, the tissue was evaluated for any acute and chronic inflammation as well as for a histiocytic and foreign-body giant-cell reaction. The macrophages were identified by immunostaining the sections with the anti-CD 68 macrophage marker (Dako) using an indirect immunoperoxidase technique. The positive cells were stained immunologically with a red color using the Avidin-biotin complex method.
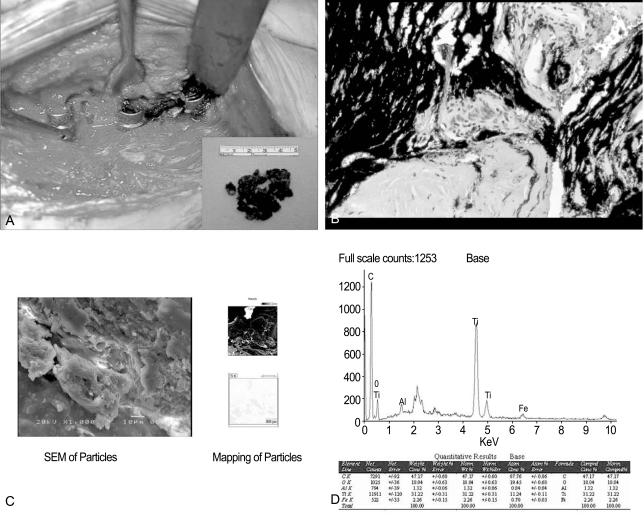
(A) Operative finding shows local discolorization of the soft tissues around spinal implant (Case 2). (B) Histologic finding reveals obvious metallosis with black staining of the tissue. (C) Scanning electron microscopy view of specimen shows the metallic debris within tissue(×1,000) and mapping of the metallic particles shows the distributions of the titanium in the tissue. (D) Quantitative analysis of the metallic debris of specimen was done with energy dispersive X-ray spectrometer.
For the scanning electron microscopy (SEM) examination in 7 cases, the specimens were fixed in 2% glutaraldehyde in a phosphate buffer and were post fixed with 1% osmium tetroxide in the same buffer and embedded in araldite. The specimens provided as a sample were cut to a size of 1×1 ×1 mm, freeze-dried for 8 hours, and subsequently coated with gold (Au) for 30 seconds. The metal debris was examined and photographed by SEM at an acceleration voltage of 20 kV (JSM 5400; JEOL Co., Tokyo, Japan). In addition, the composition distribution obtained from the SEM images was analyzed by elemental mapping (Fig. 1C). Electron dispersion X-ray analysis of the particles within the selected samples was performed to confirm the presence of metal particles in cellular samples (Fig. 1D).
After removing the spinal implants, the changes of back pain were evaluated by visual analog scale (VAS) scores and the assessment of spinal fusion was done according to Lenke's14 criteria.
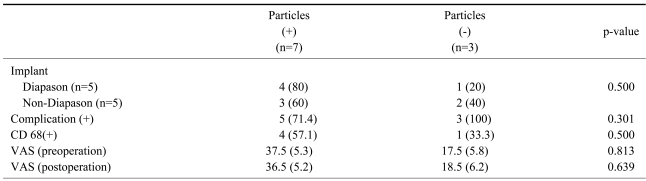
The associations between metal particles and implants, complications such as radiological abnormal findings, anti-CD 68 marker, the changes of back pain using the visual analog scale were assessed with the Mann-Whitney U-test. The histological anti-CD 68 marker was examined to determine if it was associated with the changes of back pain. All the data were compared statistically using the Mann-Whitney test. A p value <0.05 was considered significant.
Results
Ten patients were enrolled in the study. Eight patients had radiologically abnormal findings such as a periosteal reaction surrounding the pedicle screw and a screw or rod failure. Two patients in the study had late operative site pain without any radiological problems. All cultures taken intraoperatively were negative. Local discoloration of the soft tissues around metal-metal junctions was observed (Fig. 1A, Case 2), and when the metal was examined after explantation, discoloration at the screw-rod junction was identified in 7 cases. Of the 10 patients, 9 (90%) showed successful relief of back pain after removing the spinal implants. The average VAS scores improved from 6.6 prior to removal, to an average of 4.9 at the last follow-up (mean follow-up after removing spinal implants: 17 months). Radiographic fusion success according to Lenke's criteria was demonstrated in 9 cases. The radiographic fusion pattern was as follows: definitely solid in 7 cases, possibly solid in 2 cases and probably not solid in 1 case. One case complained of persistent back pain (VAS; 7) with pseudoarthrosis. Radiographic findings revealed screw breakage and pseuoarthrosis. The author recommended treatment for pseudoarthrosis but the patient rejected any further procedures except for the removal of the spinal implant.
There was obvious metallosis with black staining of the internal membrane of the tissue around the spinal implant in 7 cases (Fig. 1B). Histological finding observed mild chronic inflammation surrounding the deposition of the metal particles such as plasma cells in 3 patients and lymphocyte cuff in 2 patients. There was no acute inflammation in these specimens. The anti CD 68 positive macrophages were observed at tissue adjacent to the metal particles in 5 patients (Fig. 2, Case 5) in contrast to stainless steel implants8. We did not verify a positive association between the deposition of the metal particles and anti-CD 68 macrophage marker.
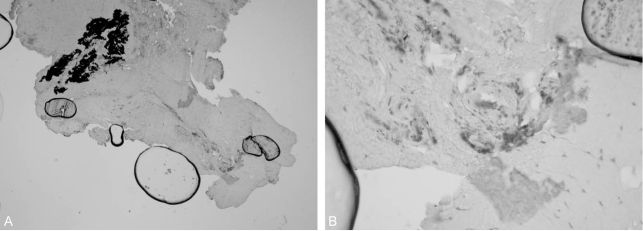
(A) Metallic debris was identified in the dense connective tissue and the anti Cotrel-Dubousset 68 positive macrophages were observed at tissue adjacent to the metal particles (Avidin-biotin complex, ×100). (B) Macrophages as stained positive by anti CD 68 marker (Avidin-biotin complex, ×200, Case 5).
SEM of the specimen showed metallic debris within the tissue, and elemental mapping of the metallic particles showed titanium distributed in the tissue of 5 cases (Fig. 1C). Electron dispersion X-ray analysis showed that the black particles forming clusters were rich in titanium (Fig. 1D).
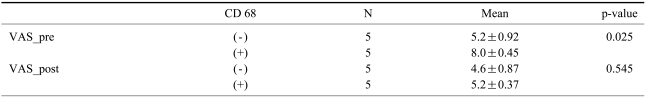
Comparison of radiological complications and metal particles showed no significant differences in the generation of the metal particles (p=0.30). There were no statistically significant differences in the association between metal particles and anti-CD 68 marker as well as the association between metal particles and the visual analog scale (p>0.05). The occurrence of the metal particles was unrelated to radiological abnormal findings (p>0.05) (Table 2). Comparison of the anti-CD 68 positive group (n=5) and the anti-CD 68 negative group (n=5) showed significant difference in back pain preoperatively (p=0.025) and no difference in back pain postoperatively (p=0.545) using the Mann-Whitney U-test (Table 3). Improvement of back pain may be associated with macrophage response rather than the metal particles.
Discussion
Titanium alloy, containing approximately 90% titanium, 6% aluminum, and 4% vanadium, is a light and strong metallic material that is highly biocompatible and commonly used in orthopedic practice15,16. Titanium is generally regarded as safe for an organism. However, it has been reported that titanium has the biochemical activity of increasing the prostaglandin E2 or interleukin-117,18. Titanium is known to wear out easily, and may cause localized problems in the tissues surrounding the implants, or travel to distant organs and cause systemic problems19,20.
Metallic particles from titanium alloy spinal implants were examined metallurgically and histologically in this study. Metallic particles were found abundantly around the intact screw-rod interface, as well as at the site of the screw or rod failure. SEM of the specimen showed metallic debris within the tissue, and mapping of the metallic particles showed titanium distributed in the tissue in 5 cases. The results of immunohistochemical staining revealed macrophages to be mainly present in the soft tissues adjacent to the metal particles. No signs suggesting an allergic reaction or infection were found on histological examination. These findings show that titanium particles had been generated by the titanium-alloy pedicle screw constructs and deposited in the soft tissue immediately surrounding the instrumentation, and the reaction against the metallic particles was manifested by the migration of macrophages to the involved area. A review of the joint prosthesis literature revealed articles describing local tissue reaction to metallic wear debris21-24, as well as in vitro human macrophage response to retrieved titanium alloy particles25,26. According to Wang et al.12, metallic particles from titanium alloy spinal implants activated a macrophage cellular response in the spinal tissues similar to that observed in joint prostheses. A number of other retrospective clinical studies have documented an inflammatory, foreign-body reaction in the soft tissue structures adjacent to spinal implants27-30. Cook et al.6 reported that late operative site pain is most likely caused by local soft tissue reaction to the implants, and late operative site pain of no apparent cause after posterior instrumentation of scoliosis is a distinct clinical entity and is relieved by implant removal in most patients. This study demonstrated that 9 patients (90%) had successful relief of their back pain after removing spinal implants.
Limitations of this study include relatively small material samples, limited cases10, and the lack of a control group. Also, it was assumed that metal particles were continuously generated from micromotion or macromotion between the titanium-alloy pedicle screw construct junctions for the duration of implantation. Metallic particles from titanium alloy spinal implants could activate a macrophage cellular response that might have deleterious effects on the fusion mass, spinal tissues, and neural elements. Because the effects of metal particles on the local and systemic tissues are still unknown, further investigation is needed to clarify the toxicological importance of metal particles generated from titanium alloy spinal implants.
Conclusions
Titanium particles were generated by titanium-alloy pedicle screw constructs and deposited in the soft tissue immediately surrounding the instrumentation. The presence of metallic particles released from the titanium alloy spinal implants might serve as the impetus for late-onset inflammatory response and late operative site pain.