|
 |
- Search
| Asian Spine J > Volume 12(1); 2018 > Article |
|
Abstract
We analyzed dose-dependent effects of vancomycin on wound infection bacteria and investigated the relationship between dose and microbial imbalances in patients treated with intrawound vancomycin powder during spine surgery. Numerous trials have confirmed that using intrawound vancomycin powder during spine surgery may decrease postoperative wound infection rates. However, potential risks include changes in wound infection bacteria, inhibition of bone fusion, and systemic toxicity. We searched PubMed for articles published since October 2016 with the following terms: ŌĆ£local vancomycinŌĆØ or ŌĆ£intrawound vancomycinŌĆØ or ŌĆ£intraoperative vancomycinŌĆØ or ŌĆ£intrawound vancomycinŌĆØ or ŌĆ£topical vancomycinŌĆØ and ŌĆ£spinal surgeryŌĆØ or ŌĆ£spine surgery.ŌĆØ We also screened the reference lists of included articles for additional studies and extracted data related to dose, infecting bacteria, sample size, infection rate and types, location of spine surgery, and perioperative antibiotics used. Our review includes one prospective and nine retrospective studies. Overall, 1 or 2 g local vancomycin powder was used in 2,394 patients. Gram-negative bacteria were dominant in patients in whom 1 g vancomycin powder was used, whereas gram-positive bacteria were dominant in those in whom 2 g powder was used. The exact mechanism underlying this dose-dependent trend remains unclear, although it may be attributed to the pharmacological characteristics of vancomycin. The included studies showed that trends in infection bacteria may change after the use of topical vancomycin powder. In addition, the observed increase in gram-negative bacteria when intrawound vancomycin powder is used has generated considerable attention. The present results differ from previous results but do not provide additional information regarding vancomycin dose and microbial changes in infected wounds. Additional large randomized controlled trials are needed to determine the relationship between vancomycin dose and the types of wound infection bacteria in patients treated with intrawound vancomycin powder during spine surgery.
The use of intrawound vancomycin powder during spine surgery to prevent postoperative wound infection is considered to pose no risks. Many articles, including systematic reviews, meta-analyses, and clinical trials, have confirmed that using intrawound vancomycin powder during spine surgery could provide considerable benefits with few side effects [12345678]. However, some in vitro, animal, and human studies suggest that vancomycin powder inhibits the osteoblast and dural cell viability [91011]. Furthermore, some studies have shown that using vancomycin intraoperatively has potential risks, including dose-dependent inhibiting effects and changes in microbial trends [10121314]. One retrospective study revealed that the intraoperative microbial trends in surgical site infections appeared to be different in patients in whom intrawound vancomycin powder was used during spine surgery than those in patients who did not; however, the investigators did not examine the relationship between the vancomycin dose used and types of infection bacteria [12]. Therefore, we conducted a systematic review of articles that reported results of previous studies on wound infection that reported the characteristics of infection bacteria in patients in whom local vancomycin powder was used. We also analyzed the relationship between the dose used and types of infection bacteria.
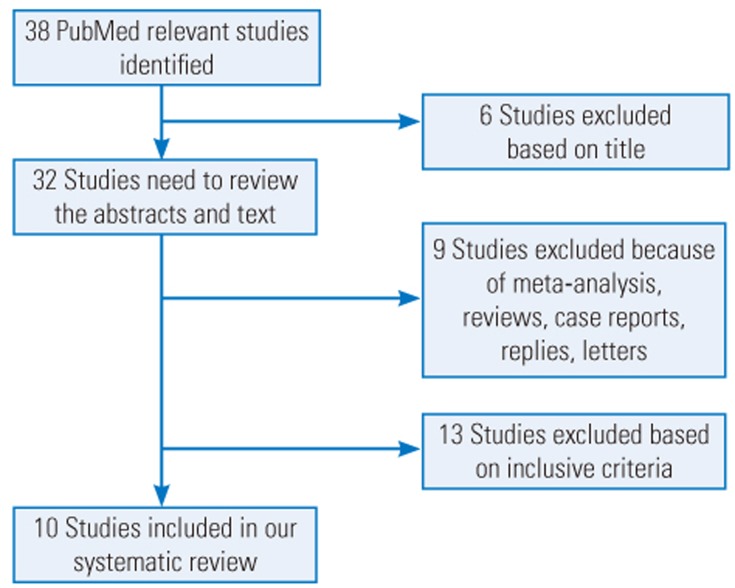
We searched PubMed for articles published since October 2016 using the combination of the following terms: ŌĆ£local vancomycinŌĆØ or ŌĆ£intrawound vancomycinŌĆØ or ŌĆ£intraoperative vancomycinŌĆØ or ŌĆ£intrawound vancomycinŌĆØ or ŌĆ£topical vancomycinŌĆØ and ŌĆ£spine surgeryŌĆØ or ŌĆ£spinal surgery.ŌĆØ Data related to dose, types of infection bacteria in the study groups, sample size, infection rate and types, location of the spine surgery, and perioperative antibiotics used were extracted from the included articles (Table 1). Moreover, the reference lists of the included articles were screened to determine whether any of the references could be included in this systematic review. Two independent surgeons (ZJ and XL) performed the search and identified the articles that met the inclusion and exclusion criteria. A senior surgeon (PD) was consulted to make the final decision in cases of disagreement. A flow diagram of the literature search is shown in Fig. 1.
Inclusion criteria were human trials, studies that included the actual dose used, and studies that described the wound infection bacteria. Letters, case reports, replies, and basic or animal studies were excluded. Studies lacking a control group or the exact vancomycin dose used were also excluded (Table 2). We also collected data related to publication date, investigators, type of study, characteristics of infection bacteria in the treatment groups, dose used, number of patients, location of surgery, wound infection, and type of wound infection.
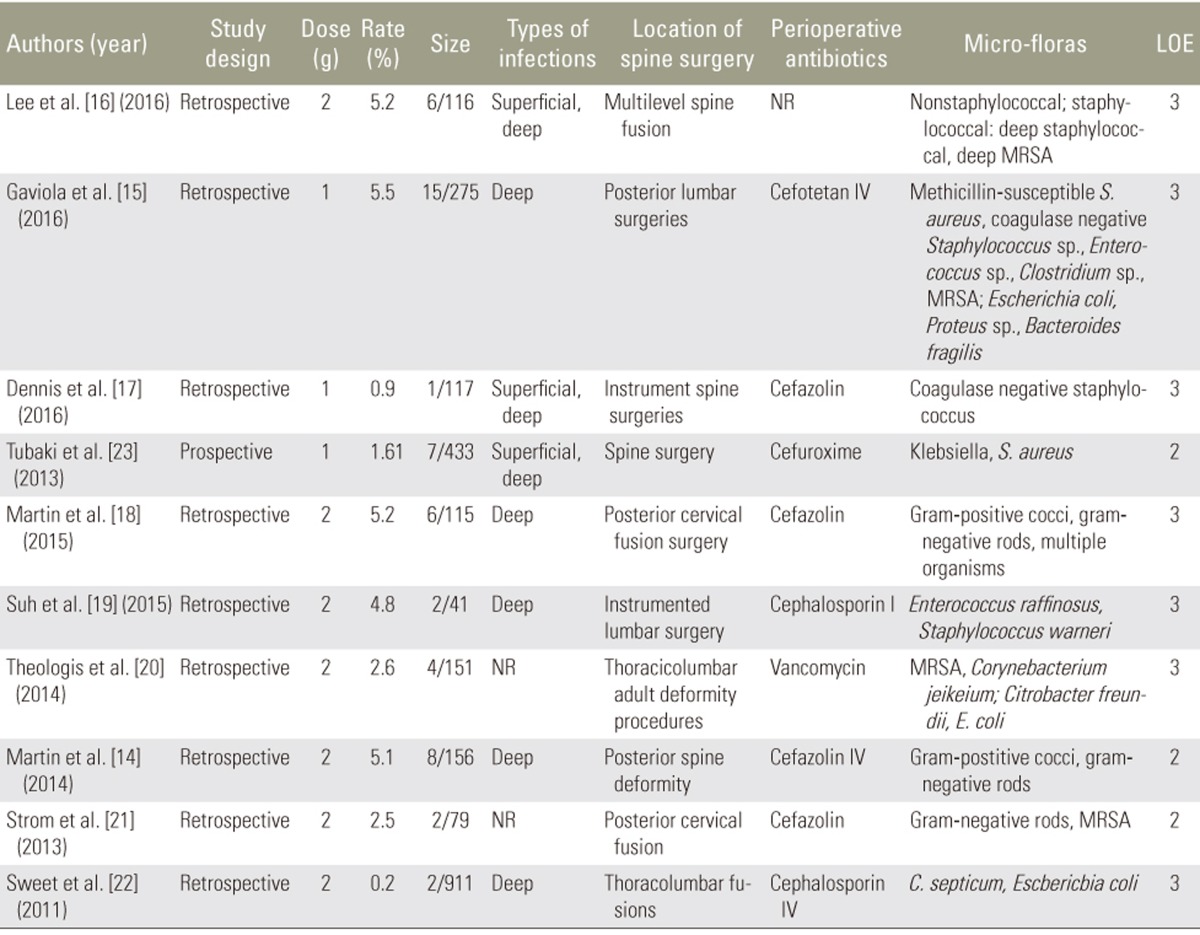
After completing our literature search, nine retrospective studies [141516171819202122] and one prospective study [23] met the inclusion criteria (Fig. 1). The comprehensive messages such as the demographic data, dose, method of application, and so on, were gathered by two independent surgeons (Table 2). Results of the analysis suggested a complicated relationship between vancomycin dose and trends among microorganisms causing the infection in the vancomycin group. Overall, 1 or 2 g local vancomycin powder was used in 2,394 patients, and the total postoperative wound site infection rate was 2.21% (53 of 2,394 patients). Many bacteria were cultured from the postoperative wound drainage in the 53 patients. Gram-negative bacteria were predominant in the intervention group wherein 1 g vancomycin powder was used. In contrast, gram-positive bacteria were predominant in the group wherein 2 g vancomycin powder was used. Only one retrospective study evaluated the characteristics of the wound infection bacteria following application of intrawound vancomycin powder. However, the investigators did not examine dose-dependent microbial changes. Our systematic review results did not provide any evidence related to the exact mechanism underlying the dose-dependent trends of these microorganisms. The most likely mechanism may be attributed to the pharmacological characteristics of vancomycin powder. Thus, the lack of randomized controlled studies containing sufficient sample sizes to explore the relationship between the dose and secondary infection in patients treated with intraoperative vancomycin powder may have caused a misunderstanding with respect to aspects such as which method of administration is suitable or whether there is dysbacteriosis from the infected wound in patients in whom intrawound vancomycin powder is used.
The efficacy of using intrawound vancomycin to prevent postoperative wound infections following spine surgery has been confirmed by previous studies, and most investigators have reported no side effects that could be attributed to the local application of vancomycin powder. With regard to the incidence of postoperative wound infections following various spine surgeries, excluding those for spine tumors, no significant differences between the intervention and control groups have been confirmed. At the same time, pharmacological theory and clinical studies have indicated that a 1,000-fold greater local concentration of vancomycin powder can exist in the wound without any harmful systemic effect, and the intrawound concentration was greater than the minimum inhibitory concentration (MIC, 2 ┬Ąg/mL) for methicillin-resistant Staphylococcus aureus (MRSA) [22]. However, the potential risks cannot be ignored, although no clinical complications that could be viewed as direct side effects in a substantial number of people treated with vancomycin powder during spine surgery have been reported. After reviewing the current literature, we identified the following potential risks and side effects of the prophylactic administration of intrawound vancomycin powder that should be considered [910].
It is accepted that administration of local vancomycin powder can reduce the incidence of postoperative wound infections without causing any side effects. This protective effect in wound site could be attributed to a high drug concentration in the operative incision with undetectable systemic toxic concentrations in the bloodstream, which inhibit the bacteria or kill the bacterial growth. Sweet et al. [22] analyzed vancomycin levels in the serum and wound on postoperative days 0ŌĆō3. The local levels were up to a 1,000-fold higher than MIC for MRSA, whereas the systemic levels were undetectable. No complications or adverse outcomes that could be directly attributed to the local application of vancomycin were identified. Thus, Sweet et al. [22] concluded that local administration of vancomycin powder can reduce the postoperative wound infection rate following posterior instrumented thoracolumbar spinal fusion. In addition, potentials risks, such as pseudarthrosis, cerebrospinal fluid leakage, hypotension, and renal toxicity, were not observed in the population receiving intrawound prophylaxis with vancomycin powder. Other clinical trials also indicated that the local application of prophylactic vancomycin powder did not lead to side effects or clinical complications [1516172021]. However, an in vitro study performed by Eder et al. [13] strongly suggested that topical administration of vancomycin powder influences bone healing in spinal fusion. In addition, Goldschmidt et al. [10] confirmed that use of local vancomycin can induce human dural cell death, inhibit growth, and alter cellular morphology in a concentration-dependent manner. Mariappan et al. [24] speculated that the absorption of local vancomycin powder caused an anaphylactic reaction resulting in circulatory collapse in one patient in whom 1 g vancomycin powder was used during spine surgery. Although the studies included in our systematic review suggested that local administration of vancomycin powder during spine surgery did not generate side effects or clinical complications, the investigators also emphasized caution when using topical vancomycin for this purpose. Therefore, the safety of intrawound vancomycin powder used during spine surgery should be verified by a substantial number of in vitro and clinical experiments.
Applying intrawound vancomycin powder during spine surgeries can change microbial trends among wound infection bacteria. Microbial changes were observed in human trials (level of evidence [LOE] 4) and documented in a retrospective case series of 981 consecutive patients in whom mean 1.13 g (range, 1ŌĆō6 g) of vancomycin powder was used during various spine surgeries. Ghobrial et al. [25] reported an increased prevalence of gram-negative (60% versus 21%, p=0.0001) and polymicrobial (19% versus 15%, p=0.9638) wound infections. The gram-negative bacteria included Serratia marcescens, Enterobacter aerogenes, Bacteroides fragilis, E. cloacae, Pseudomonas aeruginosa, and Citrobacter koseri. Most positive cultures were from the lumbar spine (n=35, 67%), followed by the thoracic (n=10, 20%) and cervical (n=6, 13%) spine. However, we did not investigate the details related to the relationship of the local vancomycin powder dose and type of wound infection bacteria. Our review revealed a pronounced difference between the two subgroups.
With regard to the types of wound infection bacteria in the populations that received prophylactic intrawound vancomycin powder, there were obvious differences between the groups wherein 1 and 2 g powder was used. The numerous inclusion and exclusion criteria used in this systematic review resulted in 10 studies to include in our review. These studies were divided into two subgroups based on whether they used 1 or 2 g intrawound vancomycin powder.
Three articles were included in the 1 g vancomycin group: two had a LOE of 3 [1517] and one had a LOE of 2 [23]. The postoperative wound infection rates were 1.61% (7 of 433 ), 0.9% (1 of 117), and 5.5% (15 of 275) in the studies by Tubaki et al. [23], Dennis et al. [17], and Gaviola et al. [15], respectively. The primary infection bacteria were gram-positive microbes (Fig. 2, Table 2). It is noteworthy that the intravenous (IV) antibiotics used in the perioperative period were different (cefuroxime, cefazolin, and cefotetan, respectively).
It is unclear whether the use of intrawound vancomycin powder during spine surgery can change microbial trends among bacteria. Coagulase-negative Staphylococcus sp. were confirmed by Dennis et al. [17] in the treatment group (1 g IV cefazolin and 1 g topical vancomycin powder). In that retrospective cohort comparative study, among all patients who underwent instrumented spine surgery at a single institution, Pseudomonas sp. (35.2%) and MRSA (22%) were found in the control group where in 1 g IV cefazolin alone was used. That study concluded that P. aeruginosa was the most common organism detected in the vancomycin group. Thus, Tubaki et al. [23] revealed two S. aureus and one Klebsiella sp. infections in the control group compared to one S. aureus and two Klebsiella sp. infections in seven patients who suffered a bacterial infection, including patients in the vancomycin group in whom 1 g intrawound vancomycin powder was placed directly on the muscle, fascia, and subcutaneous tissues without exposing the bone graft or dura (1 g local vancomycin powder plus standard cefuroxime). These results seemed to demonstrate few changes in microorganisms based on whether vancomycin was used. In contrast, coagulase-negative Staphylococcus was the only bacteria isolated from deep wound drainage in the treatment group (0.9%, 1 of 117). In a study of patients who underwent high-risk multilevel spine fusion surgery, Gaviola et al. [15] found no significant difference in the trends among bacteria detected between the groups wherein IV cefazolin plus topical vancomycin and IV cefazolin alone were used. However, the study did not address high-risk factors and changes in microorganisms and the use of vancomycin powder during spine surgery.
Seven studies included in the subgroup reported the use of 2 g vancomycin intraoperatively, including two with a LOE of 2 [1421] and five with a LOE of 3 [1618192022]. Types of operations performed in this subgroup were cervical fusion, lumbar surgery, and spine deformity surgery.
Sweet et al. [22] reported the largest retrospective comparative cohort study, with 911 patients undergoing posterior thoracolumbar instrumentation fusion or other surgeries in whom standard prophylactic IV cephalosporin and 2 g intrawound vancomycin powder were used. Two deep wound infections occurred in the vancomycin group (n=2, 0.2%), and Clostridium septicum and Escherichia coli were cultured from the wound drainage. The investigators hypothesized secondary seeding without a clear indication of the other infection site [22]. In a study concerning patients undergoing cervical fusion with the routine local application of IV cefazolin plus 2 g intraoperative vancomycin powder, Strom et al. [21] reported postoperative wound infections in 79 cases (2.5%, n=2). MRSA and gram-negative rods were confirmed by the investigators for infected populations in the vancomycin group. Lee et al. [16] reported one staphylococcal (MRSA) and four nonstaphylococcal infections in the study group (i.e., those in whom 2 g vancomycin powder plus standard prophylactic IV antibiotics were used), and they also demonstrated a statistically significant difference in the total and staphylococcal infection rates (p=0.028 and p=0.041, respectively). However, this study did not confirm differences in the wound infection bacteria between the control and treatment groups.
Notably, Martin et al. [1418] reported different rates of wound infection, with roughly similar results from cultures (gram-positive cocci and gram-negative rods) obtained from wound drainage in patients receiving IV cefazolin who underwent posterior cervical fusion surgery (5.2%, 6 of 115) and those who underwent posterior spine deformity surgery (5.1%, 8 of 156) treated with IV cefazolin in the perioperative period. In addition, multiple organisms were observed in several studies, but they were not described definitively in patients undergoing cervical fusion surgery.
Theologis et al. [20] reported a retrospective cohort analysis of 151 patients undergoing adult deformity reconstruction in whom standard prophylactic IV vancomycin plus 2 g vancomycin powder were used. Their comprehensive analysis confirmed lower costs and a lower incidence of wound infection. Unusual bacteria, such as Corynebacterium sp., MRSA, C. freundii, and E. coli, were cultured from the wound drainage. However, no additional details were disclosed because their main aim was to investigate the effectiveness of decreasing wound infection rates and the benefits of reducing health costs; the cause of the secondary infection happened to be wound infection even in patients who were treated with intrawound vancomycin powder.
Suh et al. [19] compared a treatment group (2 g vancomycin powder plus IV cephalosporin) and a control group wherein IV cephalosporin alone was used and reported no pronounced differences in the erythrocyte sedimentation rates or C-reactive protein concentrations (p=0.004) [19]. Two postoperative contaminated results of culture from the surgical drains showed Enterococcus raffinosus and S. warneri.
Thus, our findings suggested the following: the primary wound infection bacteria were gram-negative bacteria, followed by gram-positive bacteria in patients treated with 1 g local vancomycin powder during spine surgery. In contrast, gram-positive bacteria were predominant in the subgroup treated with 2 g vancomycin powder. However, the relationship between the pathogen and dose used remained unclear.
Many meta-analyses to date have provided high-level evidence that local administration of vancomycin powder can reduce the incidence of postoperative wound infection with no side effects, including secondary infection, attributable to the local vancomycin powder. However, at the same time, the investigators also emphasize underlying risks, including pseudarthrosis, deep vein thrombosis, systemic toxicity, dose-dependent effects, and changes in microbial trends.
Ghobrial et al. [25] investigated the microbial trends in postoperative spine wound infections in patients who had been treated with local vancomycin powder (average dose, 1.13 g; range, 0.5ŌĆō6 g). Regarding positive wound cultures, 51 infections were detected by means of bacterial culture in the treatment group wherein intraoperative vancomycin powder was used (infected rate, 5.2%; 51 of 981). Notably, nine patients with spine trauma showed positive results in cultures obtained from the wound drainage. The investigators reported that the incidence of gram-negative or polymicrobial infections was increasing along with the use of intraoperative vancomycin powder in spine surgery. However, few studies have investigated the relationship between demographic data and positive cultures or the dose-dependent trends of microbial contamination. Later, Ghobrial et al. [12] also investigated complications following the use of local vancomycin powder during lumbar surgery and explored the effect of dose on those complications. Based on their analysis of 14 retrospective and two prospective studies involving 9,721 patients, they revealed an increasing trend for gram-negative bacteria derived from the wound drainage, which may become an urgent problem when vancomycin powder is used intraoperatively. However, the study did not examine dose-dependent trends in microorganisms in the infected population. In addition, only lumbar surgery was included in this study.
In a Lewis rat study, Tennent et al. [26] suggested that lower rate of infections caused by S. aureus in treatment group received IV injections of cefazolin along with intrawound vancomycin powder compared with the Lewis rat accepted the IV alone after inoculated the implants with S. aureus. They explored the time-dependent effectiveness of vancomycin and provided substantial support for the local application of antibiotics in spine surgery in future studies. Subsequent trials or in vitro experiments should investigate changes in the microbial trends and topical use of vancomycin powder, particularly with regard to higher-risk populations for spine surgery. According to our analysis, the ŌĆ£double-edged swordŌĆØ of vancomycin powder's effectiveness in various spine surgeries should be considered. The possible mechanism of the dose-dependent effect of vancomycin remains complex; thus, the identification of subgroups for preventing postoperative wound infection and investigating the mechanism of the dysbacteriosis will be the challenges for future studies.
Several limitations of our systematic review should be acknowledged. Most studies that met the inclusion criteria were retrospective; only one prospective study was included in this review (a total of 2,394 patients). Only 53 patients were confirmed to have postoperative wound infections, which may have been a source of bias. Moreover, the heterogeneity of the different trials and types of perioperative IV antibiotics used in different studies may influence the rate or microfloras of postoperative wound infection in intervention groups. In addition, all of our included studies were reported only in English and were identified using only PubMed; we did not search other databases, such as Embase, Google Scholar, or Web of Science. Finally, characteristics of wound infections, rate, size, and other data for the control group were not analyzed in this article, as the main purpose was to investigate the dose-dependent changes in microbial trends in the intraoperative vancomycin group. As the above limitations show, many randomized controlled trials to examine time- or dose-dependent effects and high-risk factors relevant to vancomycin's effectiveness must be performed in the future.
Our systematic review revealed that gram-negative bacteria were predominant in the population wherein 1 g vancomycin powder was used intraoperatively. In contrast, gram-positive bacteria were predominant in patients in whom 2 g vancomycin powder was used intraoperatively. However, the mechanism underlying these dose-dependent trends remains unclear. Therefore, future randomized controlled trials with large sample sizes should explore the relationship between vancomycin dose and secondary infections in patients who experience postoperative wound infections even if treated with local vancomycin powder during spine surgery. In addition, they should also examine relationships between high-risk factors, such as the presence of diabetes mellitus and advanced age, and microorganisms in patients undergoing spine surgery. The time-dependent effectiveness of using intrawound vancomycin powder during spine surgery is also an important future research direction.
Acknowledgments
This study was supported by the Hunan Provincial Innovation Foundation for Postgraduates (CX2016B617) and the Scientific Program of the Health and Family Planning Commission of Hunan Province (C2016129).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
1. Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J 2015;15:762ŌĆō770. PMID: 25637469.


2. Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg 2015;83:816ŌĆō823. PMID: 25535069.


3. Xiong L, Pan Q, Jin G, Xu Y, Hirche C. Topical intrawound application of vancomycin powder in addition to intravenous administration of antibiotics: a meta-analysis on the deep infection after spinal surgeries. Orthop Traumatol Surg Res 2014;100:785ŌĆō789. PMID: 25281550.


4. Khan NR, Thompson CJ, DeCuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine 2014;21:974ŌĆō983. PMID: 25259555.


5. Evaniew N, Khan M, Drew B, Peterson D, Bhandari M, Ghert M. Intrawound vancomycin to prevent infections after spine surgery: a systematic review and meta-analysis. Eur Spine J 2015;24:533ŌĆō542. PMID: 24838506.


6. Alcala-Cerra G, Paternina-Caicedo AJ, Moscote-Salazar LR, Gutierrez-Paternina JJ, Nino-Hernandez LM. Application of vancomycin powder into the wound during spine surgery: systematic review and meta-analysis. Rev Esp Cir Ortop Traumatol 2014;58:182ŌĆō191. PMID: 24703108.


7. Glotzbecker MP, Riedel MD, Vitale MG, et al. What's the evidence?: systematic literature review of risk factors and preventive strategies for surgical site infection following pediatric spine surgery. J Pediatr Orthop 2013;33:479ŌĆō487. PMID: 23752143.


8. Zebala LP, Chuntarapas T, Kelly MP, Talcott M, Greco S, Riew KD. Intrawound vancomycin powder eradicates surgical wound contamination: an in vivo rabbit study. J Bone Joint Surg Am 2014;96:46ŌĆō51. PMID: 24382724.


9. Philp AM, Raja S, Philp A, Ede MP, Jones SW. The effect of vancomycin and gentamicin antibiotics on human osteoblast proliferation, metabolic function and bone mineralisation. Spine (Phila Pa 1976) 2016 5 24 PMID: 10.1097/BRS.0000000000001712. [Epub].
10. Goldschmidt E, Rasmussen J, Chabot JD, et al. The effect of vancomycin powder on human dural fibroblast culture and its implications for dural repair during spine surgery. J Neurosurg Spine 2016;25:665ŌĆō670. PMID: 27258478.


11. Mendoza MC, Sonn KA, Kannan AS, et al. The effect of vancomycin powder on bone healing in a rat spinal rhBMP-2 model. J Neurosurg Spine 2016;25:147ŌĆō153. PMID: 27035510.


12. Ghobrial GM, Cadotte DW, Williams K Jr, Fehlings MG, Harrop JS. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus 2015;39:E11.

13. Eder C, Schenk S, Trifinopoulos J, et al. Does intrawound application of vancomycin influence bone healing in spinal surgery? Eur Spine J 2016;25:1021ŌĆō1028. PMID: 25904413.



14. Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for spinal deformity surgery. Spine (Phila Pa 1976) 2014;39:177ŌĆō184. PMID: 24158179.


15. Gaviola ML, McMillian WD, Ames SE, Endicott JA, Alston WK. A retrospective study on the protective effects of topical vancomycin in patients undergoing multilevel spinal fusion. Pharmacotherapy 2016;36:19ŌĆō25. PMID: 26749522.


16. Lee GI, Bak KH, Chun HJ, Choi KS. Effect of using local intrawound vancomycin powder in addition to intravenous antibiotics in posterior lumbar surgery: midterm result in a single-center study. Korean J Spine 2016;13:47ŌĆō52. PMID: 27437012.



17. Dennis HH, Wei DT, Darren KZ, et al. Is intraoperative local vancomycin powder the answer to surgical site infections in spine surgery? Spine (Phila Pa 1976) 2016 5 23 PMID: 10.1097/BRS.0000000000001710. [Epub].
18. Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for posterior cervical fusion surgery. J Neurosurg Spine 2015;22:26ŌĆō33. PMID: 25380539.


19. Suh BK, Moon SH, Kim TH, et al. Efficacy of antibiotics sprayed into surgical site for prevention of the contamination in the spinal surgery. Asian Spine J 2015;9:517ŌĆō521. PMID: 26240708.



20. Theologis AA, Demirkiran G, Callahan M, Pekmezci M, Ames C, Deviren V. Local intrawound vancomycin powder decreases the risk of surgical site infections in complex adult deformity reconstruction: a cost analysis. Spine (Phila Pa 1976) 2014;39:1875ŌĆō1880. PMID: 25077909.


21. Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976) 2013;38:991ŌĆō994. PMID: 23324930.


22. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976) 2011;36:2084ŌĆō2088. PMID: 21304438.


23. Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976) 2013;38:2149ŌĆō2155. PMID: 24048091.


24. Mariappan R, Manninen P, Massicotte EM, Bhatia A. Circulatory collapse after topical application of vancomycin powder during spine surgery. J Neurosurg Spine 2013;19:381ŌĆō383. PMID: 23829290.


25. Ghobrial GM, Thakkar V, Andrews E, et al. Intraoperative vancomycin use in spinal surgery: single institution experience and microbial trends. Spine (Phila Pa 1976) 2014;39:550ŌĆō555. PMID: 24480966.


26. Tennent DJ, Shiels SM, Sanchez CJ Jr, et al. Time-dependent effectiveness of locally applied vancomycin powder in a contaminated traumatic orthopaedic wound model. J Orthop Trauma 2016;30:531ŌĆō537. PMID: 27124826.


- TOOLS