|
 |
- Search
| Asian Spine J > Volume 15(3); 2021 > Article |
|
Abstract
Notes
Author Contributions
Conceptualization and writingŌĆōoriginal draft preparation: Sabino Luzzi, Cristian Gragnaniello; soft ware and valida-software and validation: Stefano Marasco, Alice Giotta Lucifero; review and editing: Mattia Del Maestro; validation: Giuseppe Bellantoni; and supervision: Renato Galzio.
Acknowledgments
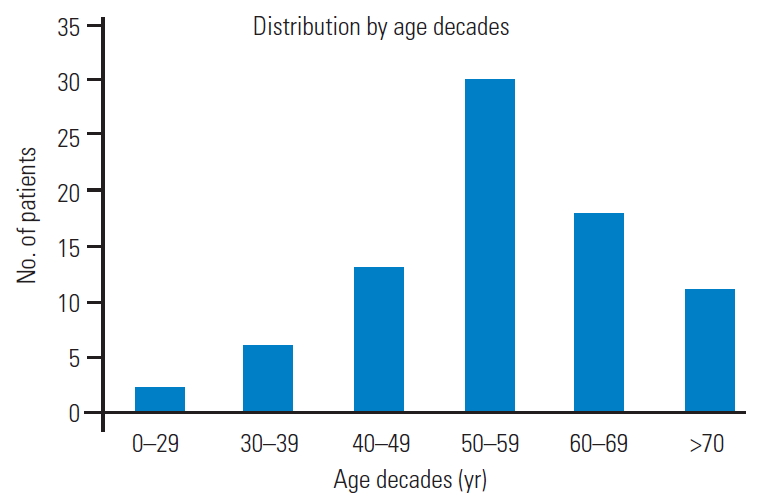
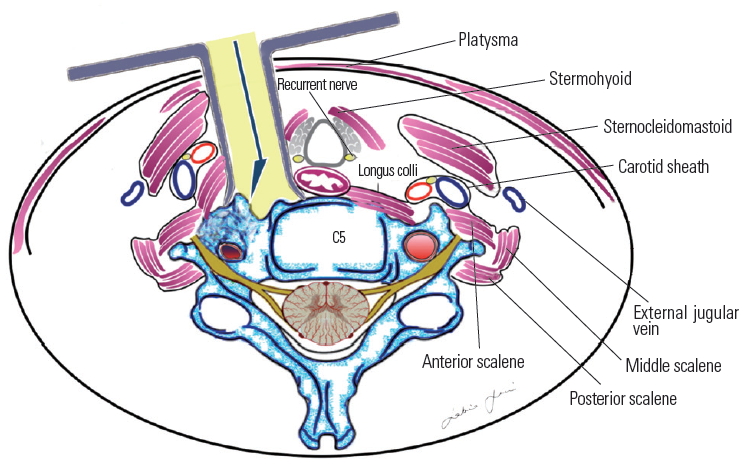
Fig.┬Ā4.
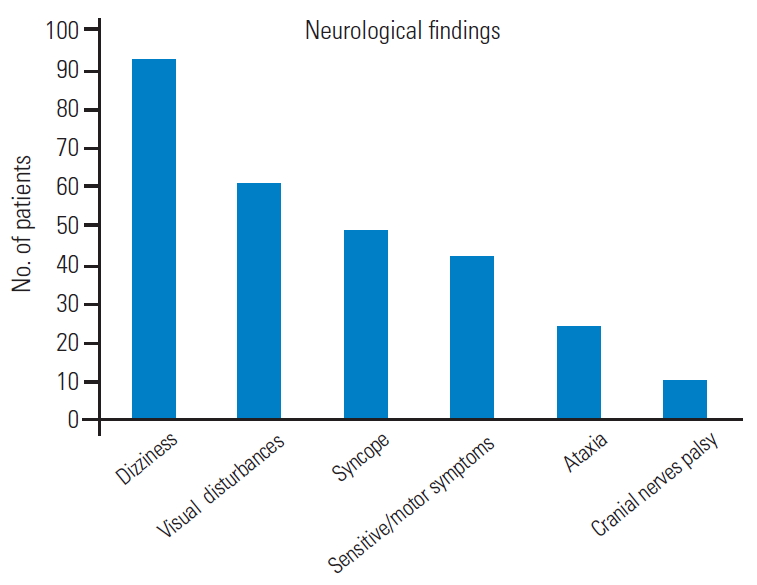
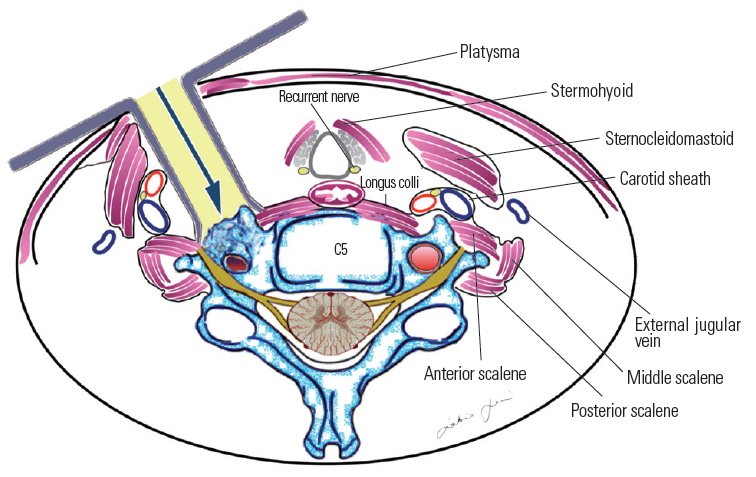
Fig.┬Ā7.
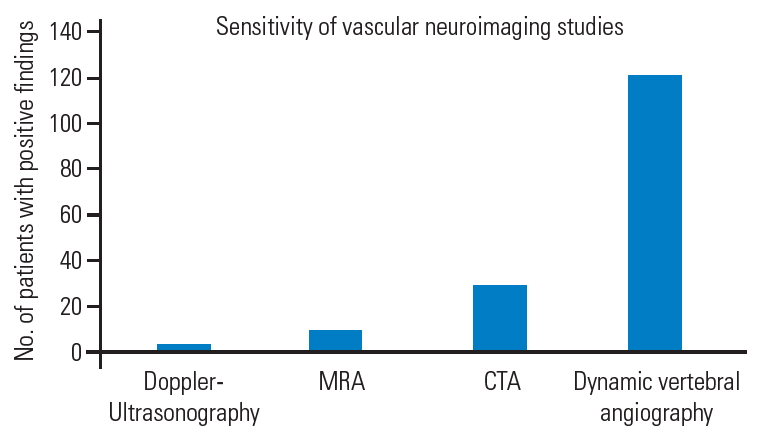
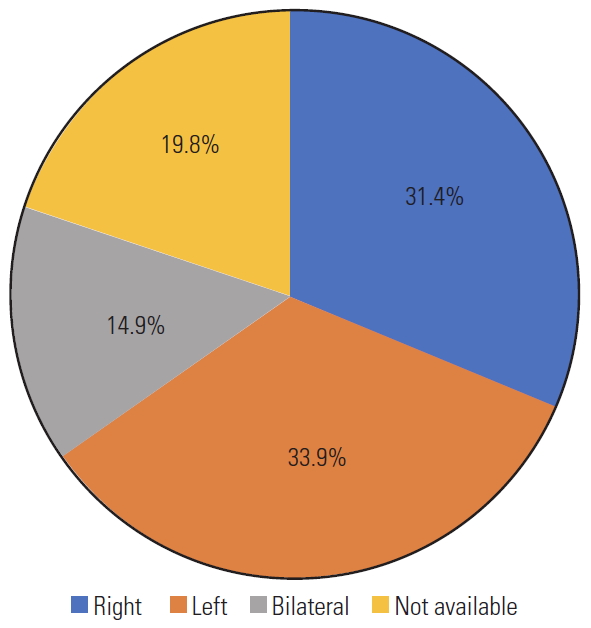
Fig.┬Ā10.
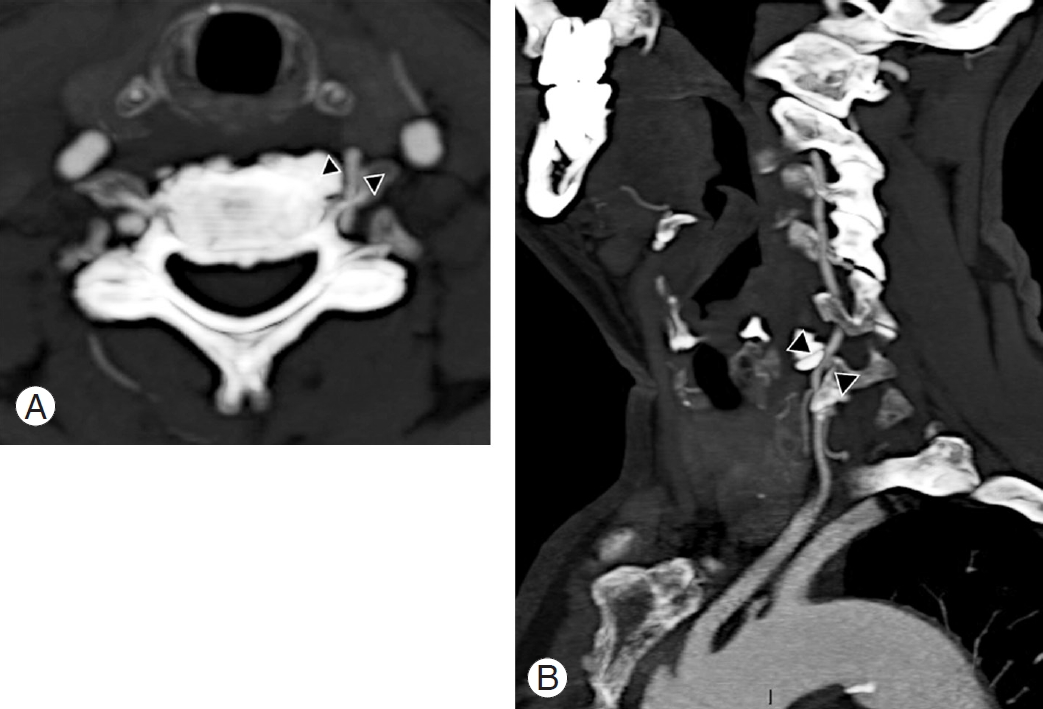
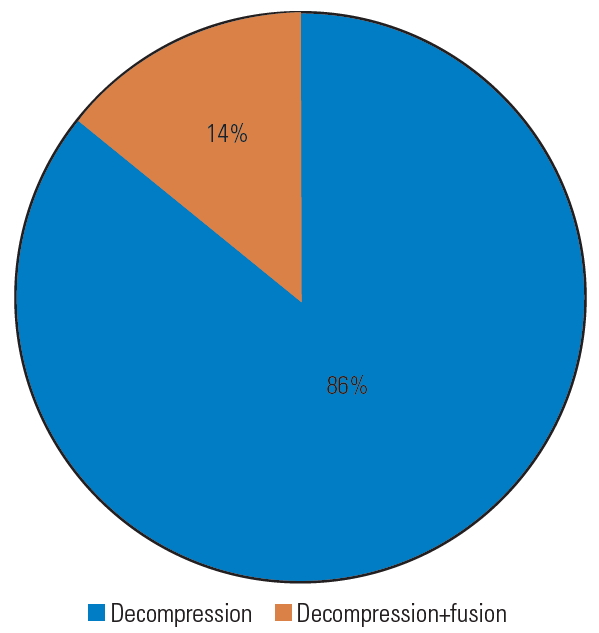
Fig.┬Ā11.

Fig.┬Ā12.
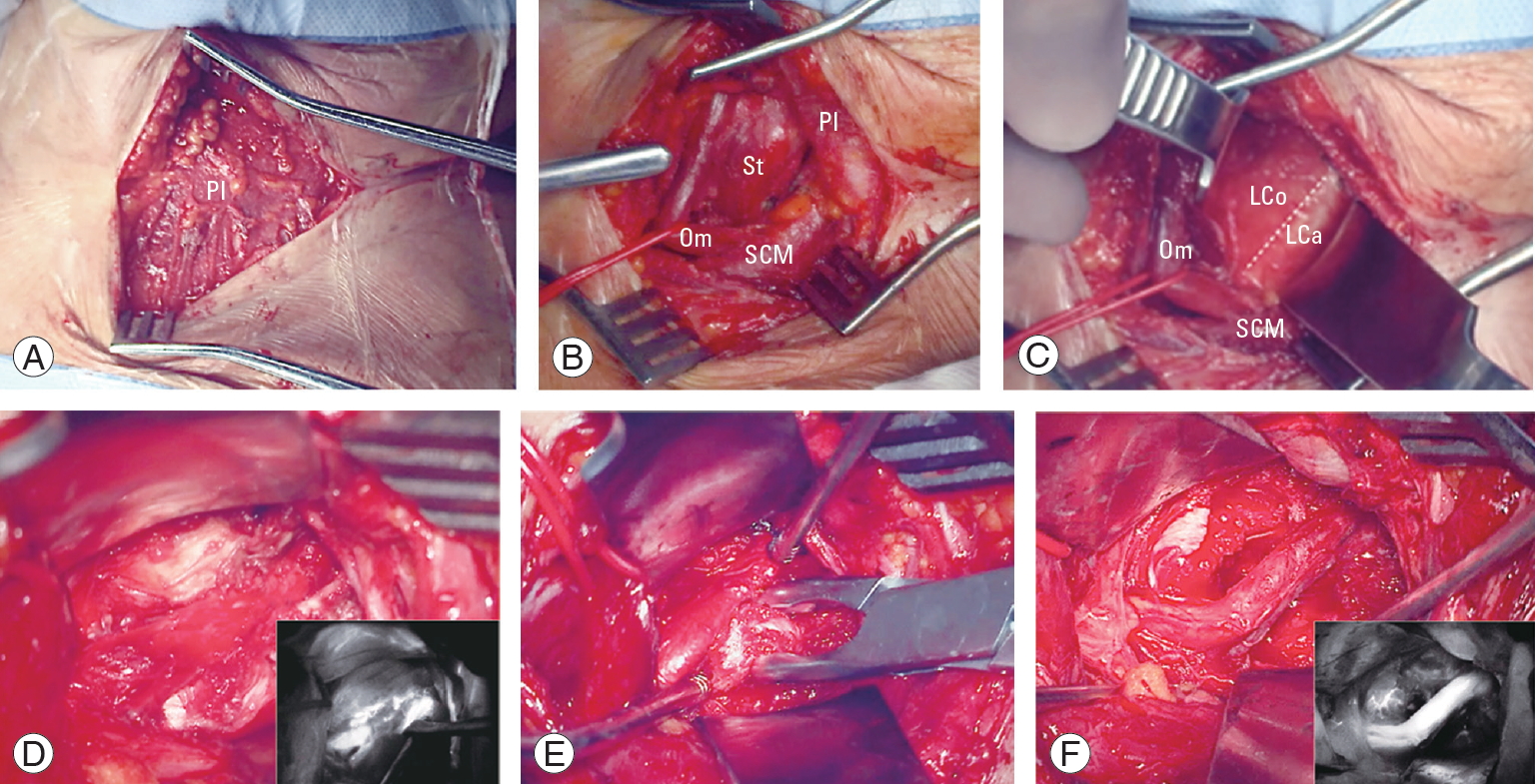
Fig.┬Ā13.
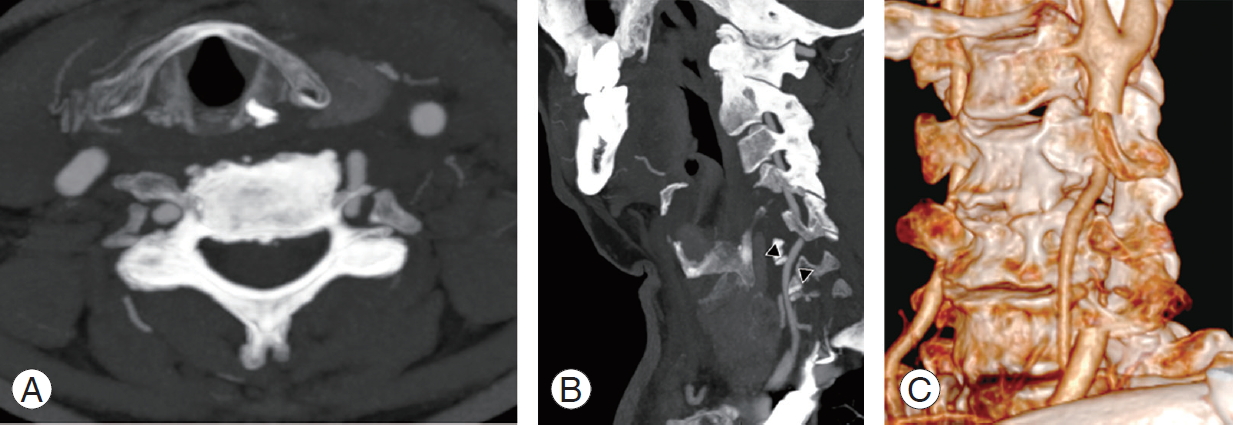
Fig.┬Ā17.

Table┬Ā1.
| No. | Authors (year) | No. of cases | Average age (yr) | Sex | Risk factors for stroke | Symptoms | Trigger | Neuroimaging techniques | Compression site | Laterality | Etiology and contributing factors | Treatment | Postoperative imaging | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Powers et al. [41] (1961) | 17 | NA | NA | None | Dizziness, visual disturbances, sensitive symptoms | Ext, Rot | dynAG | NA | NA | Muscle band | ASR (AA) | dynAG | Improved |
| 2 | Gortvai [16] (1964) | 1 | 56 | M | HP | Dizziness, syncope, hemiparesis, cervicalgia | E xt, Lt/Rt Rot | XR, dynAG | C6 | Lt | SPL, CST, DH, FR | ATR, OR, (AA) | dynAG | Improvement of hemiparesis |
| 3 | Bakay and Leslie [18] (1965) | 1 | 47 | M | HP, DM | Dizziness, syncope, paresthesia | Lt Rot | XR, dynAG | C5 | Rt | SPL, SPLS | OR, F (AA) | dynAG | Mild dizziness |
| 1 | 42 | M | None | Dizziness, cervicalgia | Ext, Rt Rot | XR, dynAG | C6 | Rt | SPL | ACDF (AA) | dynAG | Complete resolution | ||
| 4 | Hardin [17] (1965) | 9 | 63 | M (6); F (3) | HP (3) | Visual disturbances (7); sensitive/motor symptoms (6); dizziness (5); syncope (4); dysarthria (4); tinnitus (2); ataxia (2) | Rot | dynAG | C2ŌĆōC6 (1); C5 (3); C6 (4) | NA | SPL | FT (AA) | dynAG | Complete resolution (5); tinnitus, deafness (1); dizziness, paresis (1); hoarseness (1); hemianopsia (1) |
| 5 | Husni [42] (1967) | 23 | 40 | M (13); F (10) | None | Dizziness (23); gait disturbances (12); syncope (6); visual disturbances (6); sensitive symptoms (4) | Rot | dynAG | C6 | Rt (6); Lt (10); bilateral (7) | LC (10); LC+AS (8); AS (4); MS (1) | Decompression (AA) | dynAG | Complete resolution (22); improved (1) |
| 6 | Nagashima [19] (1970) | 20 | 51 | M (12); F (8); | HP (4) | Dizziness (17); visual disturbances (12); syncope (10); cranial nerve palsy (8); sensitive/motor symptoms (8); ataxia (7); nystagmus (3) | Rot | dynAG | C 4 (5); C5 (12); C6 (4) | Rt (9); Lt (6); bilateral (5) | SPL, FR | OR, FT (AA) | XR, dynAG | Complete resolution (12); improved (6); suicide (1); myocardial infarction (1); |
| 7 | Smith et al. [20] (1971) | 1 | 51 | F | None | Syncope, ataxia, headache | Ex | XR, dynAG | C5 | Lt | SPL, CST | D, OR, F (AA) | dynAG | Complete resolution |
| 1 | 53 | M | None | Dizziness, hemifacial hypesthesia, paresis | Ex, Rot | XR, dynAG | C5, C6 | Bilateral | SPL | FT, OR, (AA) | dynAG | Complete resolution | ||
| 8 | Sullivan et al. [21] (1975) | 1 | 37 | M | None | Hemianopsia, headache | Rot | dynAG | C5 | Lt | SPL, FR | OR, F (AA) | dynAG | Improved |
| 9 | Dadsetan and Skerhut [43] (1990) | 1 | 56 | M | None | Dizziness, loss of vision, nystagmus | Rt Rot | CT, DSA | C7 | Lt | Muscle band | ASR, FT (AA) | DSA | Complete resolution |
| 10 | Budway and Senter [44] (1993) | 1 | 45 | M | HP | Dizziness, visual disturbances | Rot | XR, CT, MRI, dynAG | C5 | Lt | DH | D, ATR (AA) | NA | Complete resolution |
| 11 | Chin [45] (1993) | 1 | 55 | M | None | Dizziness, ataxia, tinnitus | Rot | CT, MRI, dynAG | C4 | Lt | SPL | Decompression | NA | Complete resolution |
| 12 | Kawaguchi et al. [23] (1997) | 1 | 56 | M | None | Amaurosis, tinnitus | Rt Rot | XR, CT, MRI, CTA, dynAG | C5 | Rt | SPL, FR | OR, FT (AA) | dynAG | HornerŌĆÖs syndrome, weakness |
| 13 | Kuether et al. [46] (1997) | 1 | 49 | M | None | Dizziness, presyncope | Ext, Rt Rot | DSA | C4ŌĆōC5; C2 | Rt, Lt | Left VA occlusion at C2 site | FT (PA); refused surgery on other side | NA | Not improvement |
| 1 | 62 | M | None | Presyncope | Ext, Rt Rot | DSA | C2 | Lt | SPL | Refused | NA | NA | ||
| 1 | 39 | M | None | Tinnitus, loss of vision | Rt Rot | DSA | C7 | Rt | NA | Conservatively | NA | NA | ||
| 14 | Citow and Macdonald [47] (1999) | 1 | 69 | M | None | Dizziness, dysarthria, tetraparesis, diplopia | Ext, Rt Rot | CT, MRI, DSA | C6 | Lt | SPL, FR | FJR, OR (PA) | DSA | Complete resolution |
| 15 | Kimura et al. [48] (1999) | 1 | NA | NA | None | Dizziness, presyncope, paresthesia | Rt Rot | XR, MRI, dynAG | C5ŌĆōC7 | Rt | SPL, SPLS, DH | ACDF | dynAG | Complete resolution |
| 16 | Ogino et al. [49] (2001) | 1 | 66 | M | None | Pre-syncope, dizziness, nystagmus | Rt Rot | MRI, CTA, dynAG | C4 | Rt | SPL, FR | FT, TPR (AA) | dynAG | Complete resolution |
| 17 | Vates et al. [24] (2002) | 1 | 56 | M | None | Dizziness, syncope | Lt Rot | XR, MRI, CT, DSA, DUS | C5 | Rt | DH | D, FT (AA) | CT, DUS, DSA | Complete resolution |
| 18 | Nemecek et al. [25] (2003) | 1 | 61 | M | None | Dizziness, syncope, nausea | Ex, Lt Rot | MRA, CTA, DSA, DUS | C6 | Lt | DH | D, FT (AA) | DUS | Complete resolution |
| 19 | Vilela et al. [26] (2005) | 6 | 58 | NA | HP, HL (1) | Syncope (5); dizziness (3); visual disturbances (3); leg paresis (1); dysarthria (1) | Rt Rot (4); Lt Rot (3) | MRI, DSA, CTA | C4 (3), C5 (2); C6 (3) | Rt (3); Lt (3) | SPL (4); DH (1); SPL, DH (1); | OR (4); D (1); D, OR (1); (AA) | DUS | Complete resolution (4); dizziness (2) |
| 20 | Bulsara et al. [3] (2006) | 1 | 55 | M | HP, DM | Dizziness, syncope | Rt Rot | CT, MRI, CTA, DSA | C5 | Rt | SPL, FR | OR, D, FT (AA) | CTA | Complete resolution |
| 21 | Velat et al. [50] (2006) | 1 | 58 | M | None | Dizziness, presyncope | Lt Rot | DSA | C6 | Lt | SPL | OR (AA) | DSA | Complete resolution |
| 22 | Miele et al. [51] (2008) | 1 | 48 | M | None | Syncope | Lt Rot | CT, MRI, DSA | C5 | Lt | SPL | ACDF | NA | Complete resolution |
| 23 | Tsutsumi et a [27] (2008) | 1 | 59 | M | HP, HL | Presyncope | Lt Rot | XR, MRI, CT, DSA | C5, C6 | Bilateral | SPL, SPLS, FR | FT, ACDF (AA) | XR, DSA | Complete resolution |
| 24 | Ujifuku et al. [2] (2009) | 1 | 78 | M | None | Syncope, visual disturbances | Rt Rot | MRI, CTA, DSA | C4 | Rt | DH | ATR, D (AA) | CTA | Complete resolution |
| 25 | Lu et al. [28] (2010) | 4 | 66.5 | M (4) | None | Dizziness, syncope | Rot | DSA, CTA | C4 (1); C5 (2); C6 (1) | Rt (1); Lt (3) | DH (1); SPL (1); SPL, FR (1); | FT, D (AA) | DSA | Complete resolution |
| 26 | Lee et al. [5] (2011) | 1 | 50 | M | None | Dizziness, syncope | Rt, Lt Rot | CT, MRA, DSA | C7 | Lt | Bony abnormality | Bony spurs removal (AA) | CTA | Complete resolution |
| 1 | 28 | F | None | Dizziness, ataxia, loss of vision | Ext, Rt Rot | DSA | C7 | Rt | Bony abnormality | ATR (AA) | CTA | Complete resolution | ||
| 27 | Yoshimura et al. [29] (2011) | 1 | 64 | M | HP, HL | Dizziness, syncope | Rt Rot | XR, MRI, DSA, CTA | C3 | Rt | SPL, UVI | ACDF (AA) | XR, DSA | Complete resolution |
| 28 | Dargon et al. [52] (2013) | 1 | 53 | M | None | Presyncope, photopsia | Rt Rot | DUS, DSA | C5 | Rt | Transverse process abnormality | TPR (AA) | DSA | Complete resolution |
| 29 | Fleming et al. [31] (2013) | 1 | 54 | M | None | Dizziness, diplopia, tinnitus | Rt/Lt Rot | MRI, DSA, CTA | C4 | Bilateral | SPL | ACDF (AA) | DSA | Complete resolution |
| 30 | Pinol et al. [30] (2013) | 1 | 27 | M | None | Dizziness | Rt Rot | XR, DSA, MRA | C6 | Rt | SPL, SPLS | ACDF (AA) | XR, DSA | Complete resolution |
| 31 | Buchanan et al. [32] (2014) | 1 | 52 | M | None | Weakness | Lt Rot | XR, DUS, CTA, DSA | C4 | Lt | SPL | ACDF (AA) | CTA | Complete resolution |
| 32 | Jost and Dailey [35] (2015) | 1 | 55 | M | None | Syncope, visual disturbances | Ex, Lt Rot | MRI, MRA, DSA, CTA | C6 | Lt | DH, SPL | ACDF (AA) | NA | Improved |
| 1 | 47 | F | None | Dizziness, visual disturbances | Lt Rot | MRI, MRA, DSA, CTA | C4, C5 | Lt | DH, SPL | ACDF (AA) | NA | Neck pain, stiffness | ||
| 33 | Okawa et al. [34] (2015) | 1 | 31 | F | DM | Dysarthria, confusion | Rt Rot | MRI, MRA, DSA, CTA | C5 | Rt | DH | D, FT (AA) | DSA | Complete resolution |
| 34 | Haimoto et al. [53] (2017) | 1 | 71 | M | None | Dizziness, syncope | Ext, Rt Rot | XR, CTA, DSA | C5 | Lt | SPL, DH, Osteochondroma | Tumor removal, TPR (AA) | CTA | Complete resolution |
| 35 | Johnson et al. [6] (2017) | 1 | 42 | M | None | Hemiparesis, hemianopsia | Rt Rot | MRI, DSA, CTA | C5 | Rt | Abnormal VA course | ATR (AA) | CTA | Complete resolution |
| 36 | Nishikawa et al. [36] (2017) | 1 | 78 | F | None | Dizziness, visual disturbances | Ex, Rt Rot | MRI, MRA, DSA, CTA | C5 | Rt | SPL | ATR, OR (AL) | CTA, DSA | Complete resolution |
| 1 | 77 | M | DM | Dizziness, dysarthria | Rt/Lt Rot | MRI, MRA, DSA, CTA | C5 | Bilateral | SPL | ACDF (AA) | CTA, MRA | Complete resolution | ||
| 37 | Cornelius et al. [38] (2018) | 1 | 54 | M | None | Syncope, visual disturbances | Lt Rot | MRI, MRA, CT, CTA, DSA | C6 | Lt | SPL | FT (AL) | NA | Complete resolution |
| 38 | Iida et al. [37] (2018) | 1 | 65 | M | None | Dizziness, visual disturbances | Lt Rot | MRI, MRA, DSA, CTA | C3 | Lt | SPL | OR, F (AA) | CTA | Complete resolution |
| 39 | Ng et al. [40] (2018) | 1 | 70 | M | None | Dizziness, syncope | Lt Rot | DSA, CTA | C4 | Bilateral | SPL | ACDF (AA) | DUS, CTA | Complete resolution |
| 40 | Rendon et al. [54] (2018) | 1 | 76 | F | HP, DM, HL | Presyncope | Rt Rot | CTA | C5 | Rt | Thyroid cartilage | Decompression (AA) | CTA | Complete resolution |
| 41 | Schunemann et al. [39] (2018) | 1 | 60 | M | HP | Dizziness, syncope | Lt Rot | XR, MRI, CTA, DSA | C3 | Rt | NA | ACDF (AA) | DSA | Complete resolution |
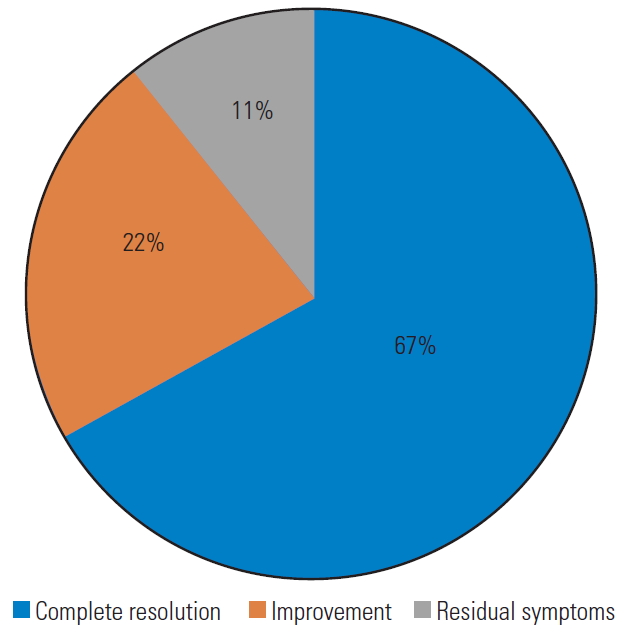
| 42 | Present case (2020) | 1 | 74 | M | HP | Dizziness, ataxia, visual disturbances, VII cranial nerve palsy | Lt Rot | XR, MRI, CT, CTA, DSA | C6 | Lt | SPL, FR | ATR, OR (AL) | CTA | Complete resolution |
NA, not available; Ext, extension; Rot, rotation; DynAG, dynamic angiography; ASR, anterior scalene resection; AA, anterior approach; M, male; F, female; HP, hypertension; Lt, left; Rt, right; XR, X-ray; SPL, spondylosis; CST, cervical spine trauma; DH, disc herniation; FR, fibrous ring; ATR, anterior tubercle removal; OR, osteophyte removal; DM, diabetes mellitus; SPLS, spondylolisthesis; F, fusion; ACDF, anterior cervical discectomy and fusion; FT, foraminotomy; LC, longus colli muscle; AS, anterior scalene muscle; MS, medium scalene muscle; D, discectomy; CT, computed tomography; DSA, digital subtraction angiography; MRI, magnetic resonance imaging; CTA, computed tomography angiography; VA, vertebral artery; PA, posterior approach; FJR, facet joint removal; PTR, posterior tubercle removal; DUS, Doppler ultrasonography; MRA, magnetic resonance angiography; HL, hyperlipidemia; UVI, uncovertebral instability; AL, anterolateral approach.