 |
 |
- Search
| Asian Spine J > Volume 14(3); 2020 > Article |
|
Abstract
Purpose
To investigate the feasibility of using fat degeneration of lumbar extensor muscle (LEM) as an alternative diagnostic criterion for sarcopenia in patients with osteoporotic vertebral fractures.
Overview of Literature
Although sarcopenia has been gaining increased attention among researchers and healthcare practitioners, there is uncertainty about the association between sarcopenia and fat degeneration of LEM.
Methods
In this study, 33 patients with osteoporotic vertebral fractures (group 1) and 29 patients without such fractures (group 2) were enrolled. Sarcopenia was diagnosed in accordance with the Asian Working Group for Sarcopenia (AWGS) criteria, including assessment of extremity muscle mass using dual-energy X-ray absorptiometry, grip strength, and gait speed. The bone mineral density and fat degeneration of LEM were investigated using magnetic resonance imaging.
Results
The mean rates of fat degeneration of LEM and the skeletal muscle index were 38.3% and 5.5 kg/m2 in group 1 and 28.9% and 6.3 kg/m2 in group 2, respectively. The fat degeneration of LEM was negatively correlated with gait speed (r=−0.44, p=0.01) and handgrip strength (r=−0.37, p=0.01). The fat degeneration of LEM also demonstrated a significant relationship with osteoporotic vertebral fractures (p=0.01). Receiver operating characteristic curve analysis between fat degeneration of LEM and osteoporotic vertebral fractures showed that the cut-off value of fat degeneration was 31.9% (sensitivity=0.67, specificity=0.66). There was a positive correlation between sarcopenia defined by the AWGS and that defined by the 31.90% cut-off value of fat degeneration of LEM instead of extremity muscle mass (r=0.46, p=0.01).
Conclusions
These results suggest the feasibility of using fat degeneration of LEM as an alternative diagnostic criterion for sarcopenia in patients with osteoporotic vertebral fractures. A cut-off value of fat degeneration of LEM of 31.9% was shown to be useful for diagnosing osteoporotic vertebral fractures.
In 2013, the Asian Working Group for Sarcopenia (AWGS) proposed diagnostic criteria for sarcopenia [1]. Quantitative methods for determining loss of skeletal muscle mass include dual-energy X-ray absorptiometry (DXA, previously DEXA), computed tomography, and magnetic resonance imaging (MRI) [2]. However, the limitation of these methods is that they are not typically amenable to the quantification of whole-body muscle mass. Diagnostic criteria for sarcopenia focus on specific individual muscles, rather than the systemic loss of muscle and its impact on overall function [3,4]. While MRI is not yet set to internationally accepted standards, researchers are interested in lumbar muscle cross-sectional area. The reason behind this is that lumbar muscle is not only an object of diagnostic imaging in degenerative disease but also reasonably well correlated with whole-body muscle mass [5,6].
The risk factors for osteoporotic vertebral fracture include thoracic kyphosis, leading to loss of spinal balance; age-related decline of muscle mass and strength (sarcopenia), which affects muscular spine stabilization; and changes in body movement patterns with advancing age, further altering spine biomechanics. In addition, the concomitant loss of osteoporosis and sarcopenia in aging spine results in poor clinical outcomes, especially repeatable osteoporotic vertebral fracture. In early 2018, the European Working Group on Sarcopenia in Older People met again to update the original definition of sarcopenia [7].
MRI evaluation has become commonly applied to patients who had an indication for their standard diagnosis. Although many studies have reported that sarcopenia is related to osteoporotic fracture, there is uncertainty about the association between sarcopenia and fat degeneration of lumbar extensor muscle as revealed by MRI. The purpose of this study was thus to investigate the feasibility of fat degeneration of lumbar extensor muscle as an alternative diagnostic criterion of sarcopenia in osteoporotic vertebral fractures.
This study enrolled 33 patients with osteoporotic vertebral fractures (group 1) diagnosed by MRI and 29 patients without such fractures (group 2) between January 2016 and December 2017. We excluded patients in whom grip strength and gait velocity could not be measured, and those with pathological fractures caused by metastatic cancer or bone tumor. The average age of the subjects in group 1 (six men and 27 women) was 78.2 years. The average age of those in group 2 (eight men and 21 women) was 74.6 years. The body mass index (BMI) was 21.9 in group 1 and 23.6 in group 2. There were no significant differences in the sex, age, or BMI of the two groups (p>0.05). The skeletal muscle index (SMI), bone mineral density (BMD), and whole-spine radiographs were also examined in the subjects. In addition, we investigated handgrip strength, gait speed, paralumbar muscle mass, and degeneration of lumbar extensor muscle by MRI. The BMD was analyzed by DXA in lumbar spine because some patients had hip arthroplasty or compressed hip screw. For the spine, the L1–L4 region was used, unless focal artifacts required the exclusion of individual vertebrae. Interpretations of the lumbar spine should always include at least two vertebrae. This study was approved by the Institutional Review Board at Kwangju Christian Hospital (IRB approval no., KCHIRB-M-2019-033), and informed consent was obtained from all individual participants included in the study.
Handgrip strength was taken using a hand dynamometer (hydraulic type; Hydraulic Hand Dynamometer Fabrication Enterprises Inc., Irvington, NY, USA) in all patients [8,9]. Handgrip strength was measured in a sitting position and with the elbow joint at 90° flexion, with the forearm away from the body at the level of the trunk. All patients were asked to apply the maximum grip strength with both left and right hands. Handgrip strength was defined as the maximally measured grip strength of the dominant hand. In the AWGS criteria, low handgrip strength is defined as <26 kg for men and <18 kg for women.
In terms of physical performance, a gait speed test was performed along an 8-m-long line. All patients were told to stand with their feet touching the starting line and were then instructed to walk at a normal speed without an assisting device. Gait speed was calculated using a smart watch [10-12]. In accordance with the criterion of the AWGS, gait speed reflective of sarcopenia was identified when it was below 0.8 m/sec.
For the diagnosis of sarcopenia, a DXA scan was performed for each patient to measure appendicular skeletal muscle for the AWGS. The SMI was considered to be reflective of sarcopenia when it was below 7.40 kg/m2 for men and 5.14 kg/m2 for women. Sarcopenia was diagnosed according to the algorithm of the AWGS [1]. AWGS recommends measuring both handgrip strength and gait speed as screening tests. The evaluation of muscle mass was subsequently performed in those with low hand grip strength and low gait speed for the diagnosis of sarcopenia.
For paralumbar muscle mass, muscle mass was calculated at the level of the L3–4 area on MRI. The MRI data were acquired on a 1.5-T Signa Excite GE (General Electric, Milwaukee, WI, USA). After scanning, the images were saved in DICOM (Digital Imaging and Communications in Medicine) file format for Picture Archiving and Communication System and were displayed and analyzed using PiView (Infinitt, Seoul, Korea) digital image viewing software. The pseudocoloring technique proposed by Lee et al. [13] was used to measure paravertebral muscle mass and fatty infiltration.
The T2-weighted axial images of the L3 spine were used. The L3 paravertebral muscles were analyzed because the L3 vertebra was at the center of the lumbar lordotic curvature, so that it could most appropriately reflect the cross-sectional area of the paravertebral muscle among the lumbar vertebrae. Moreover, since osteoporotic vertebral fracture was prevalent in the thoracolumbar junction, pseudocoloring was chosen as one of the image analysis tools. First, the region of interest (ROI) was outlined with a graphic cursor around the back extensor muscle. The fat signal intensity (in grayscale) in the muscle within the ROI was measured using a histogram and the fat signal intensity was applied to the pseudocoloring tool. In this technique, the bright pixels of the fat tissue in the magnetic resonance images were colored red using the pseudocoloring tool of the program. Subsequently, the percentage of fat infiltrated area in the paravertebral muscle (the percentage of red area in the muscle area) was determined. By using the pseudocoloring technique, multifidus muscle and erector spine muscle masses in lumbar spine MRI axial images were measured [14,15]. As a result, we obtained the paralumbar muscle mass and % fat mass. The fat degeneration could be calculated as follows: 100−muscle mass% (Fig. 1).
For statistical analyses, we used IBM SPSS for Windows ver. 20.0 (IBM Corp., Armonk, NY, USA). We evaluated the association between osteoporotic vertebral fracture and independent variables such as age, BMD, gait speed, handgrip strength, paralumbar muscle mass, and fat degeneration using a general linear model. The results are expressed as means±standard deviations. To determine the significance of relationships between osteoporotic vertebral fracture and BMD, gait speed, handgrip strength, and paralumbar muscle mass, we also performed analysis of variance. After receiver operating characteristic (ROC) curve analysis between fat degeneration of lumbar extensor muscle and osteoporotic vertebral fractures, we performed correlation analysis between sarcopenia as defined by the Asian Working Group and sarcopenia as defined by the cut-off value of fat degeneration of lumbar extensor muscle, instead of extremity muscle mass. Differences were considered to be statistically significance at p<0.05.
The mean fat degeneration of lumbar extensor muscle and SMI were 38.3% and 5.5 kg/m2 in group 1, and 28.9% and 6.3 kg/m2 in group 2, respectively. The average BMD, handgrip strength, and gait speed were −3.36 (T-score), 11.9 kg, and 0.42 m/sec in group 1 and −1.81 (T-score), 15.6 kg, and 0.61 m/sec in group 2, respectively. There were significant differences of fat degeneration of lumbar extensor muscle (p=0.01), SMI (p=0.01), BMD (p=0.01), handgrip strength (p=0.01), and gait speed (p=0.01) between the two groups (Table 1). The analysis between sarcopenia-defining criteria and fat degeneration of lumbar extensor muscle revealed negative correlations with gait speed (p=0.01) and handgrip strength (p=0.01) (Table 2).
The fat degeneration of lumbar extensor muscle demonstrated a significant relationship with osteoporotic vertebral fractures (p=0.01). ROC curve analysis between fat degeneration of lumbar extensor muscle and osteoporotic vertebral fractures showed that the optimal cut-off value of fat degeneration was 31.90% (sensitivity=0.67, specificity=0.66) (Fig. 2).
Twenty-four patients in group 1 had sarcopenia as defined by the Asian Working Group and seven patients had it in group 2. When sarcopenia was defined by the cut-off value of fat degeneration of lumbar extensor muscle of 31.90%, 22 patients in group 1 and six patients in group 2 were diagnosed with it.
There was a positive correlation between sarcopenia as defined by the Asian Working Group and sarcopenia as defined by the cut-off value of fat degeneration of lumbar extensor muscle of 31.90%, instead of extremity muscle mass (r=0.46, p=0.01).
Sarcopenia is a risk factor for osteoporotic vertebral fractures and has recently become a major geriatric healthcare problem [16]. The prevalence of sarcopenia was reported to be 5.8%–14.9% in men and 4.1%–16.6% in women, according to the International Working Group on Sarcopenia and European Working Group on Sarcopenia in Older People criteria, by using different skeletal muscle mass indices [17]. The identified prevalence of sarcopenia thus differs depending on the diagnostic criteria used.
For the evaluation of sarcopenia, the muscle mass can be determined as the total body skeletal muscle mass, appendicular skeletal muscle mass, or muscle cross-sectional area at a specific location in the body. In addition, different DXA brands have produced inconsistent results of skeletal muscle mass. MRI is considered to be the gold standard for the noninvasive assessment of skeletal muscle mass, but it is not commonly utilized in the primary evaluation because of its high cost [18].
In clinical practice, the BMD of areas such as lumbar spine and femoral neck is examined in patients of osteoporosis. In such cases, Feyerabend and Lear [19] reported that only 50% of patients had similar BMD between lumbar spine and femoral neck. When physicians face discordant results in such variables, it can be difficult to integrate these into the decision-making process. Some guidelines from the National Osteoporosis Foundation suggest that treatment should be recommended according to the T-score in the osteoporotic range, regardless of discordant BMD [20]. Thus, the diagnosis of sarcopenia could be recommended when skeletal muscle mass is in the sarcopenic range, regardless of whether discordant results have arisen in different locations. The diagnostic criterion of AWGS was applied to the appendicular muscle mass using DXA. However, the osteoporotic vertebral fracture could be affected by fat degeneration of the lumbar extensor muscle. Nevertheless, the association between fat degeneration of lumbar extensor muscle and sarcopenia is currently unknown. This study revealed the feasibility of using fat degeneration of lumbar extensor muscle as an alternative diagnostic criterion for sarcopenia for patients with osteoporotic vertebral fractures. As BMD evaluation of both lumbar spine and femoral neck has been applied for clinical purposes, skeletal mass index as a diagnostic criterion for sarcopenia, which accurately reflects the contribution of each result to fracture risk, would be preferable.
Lumbar extensor muscles have an influence on the compression fracture of vertebrae as a stabilizer. Briggs et al. [21] mentioned that intervertebral disc integrity as well as paravertebral muscle strength would prevent the spine from suffering vertebral fracture. Moreover, Ignasiak et al. [22] reported that several stages of aging and sarcopenia were modeled by identifying reduced strength of erector spinae and multifidus muscles. Based on aging from the third to the sixth decade of life, ≥60% of normal strength, sarcopenia is mild of 60% strength, moderate of 48% strength, severe of 36% strength, very severe of 24% strength. These results revealed that severe and very severe stages of sarcopenia were associated with substantial increases up to 318 N of compression at the levels of the upper thoracic spine, and up to 176 N of shear loading along the thoracolumbar spine [22]. Schlaeger et al. [23] reported that proton density fat fraction (PDFF) of MRI in erector spinae muscle could be related to muscle strength. PDFF of erector spinae muscle was shown to be a predictor of relative extensor strength of the spine [23]. In this study, ROC curve analysis between fat degeneration of lumbar extensor muscle and osteoporotic vertebral fractures showed that the cut-off value of fat degeneration was 31.9% (sensitivity=0.67, specificity=0.66). There was a positive correlation between sarcopenia defined by the Asian Working Group and sarcopenia defined by this cut-off value of fat degeneration of lumbar extensor muscle, instead of extremity muscle mass (r=0.583, p=0.01). There was also a positive correlation between fat degeneration of lumbar extensor muscle and osteoporotic vertebral fractures (r=0.46, p=0.01). We suggest that dysfunction of back extensor muscles with fat infiltration could contribute to the poor stabilizing ability of the spine, resulting in greater vulnerability to osteoporotic vertebral fracture.
This study had the limitations of a small sample size and an unbalanced sex ratio in a cross-sectional evaluation. However, despite these limitations, this study provides a foundation for future studies in this field. Further studies using a larger population and prospective design are required to clarify the feasibility of using fat degeneration of lumbar extensor muscle as an additional diagnostic criterion for sarcopenia for patients with osteoporotic vertebral fractures.
The results obtained here suggest the feasibility of using fat degeneration of lumbar extensor muscle as an alternative diagnostic criterion for sarcopenia for patients with osteoporotic vertebral fractures. The results reveal an optimal cut-off value of 31.90% for fat degeneration of lumbar extensor muscle in terms of an association with osteoporotic vertebral fractures.
Acknowledgments
We thank Dr. Choi Hyun-Soo, Department of Nuclear Medicine, for assistance with the radiographic examination.
Fig. 1.
Example of paralumbar muscle measurement using the pseudocoloring technique. (A) Magnetic resonance imaging axial image at the level of L3–4 in lumbar spine. (B) Paralumbar muscle mass (multifidus, erector spinae muscle) measurement using the pseudocoloring technique. (C) The results and values after using the pseudocoloring technique.
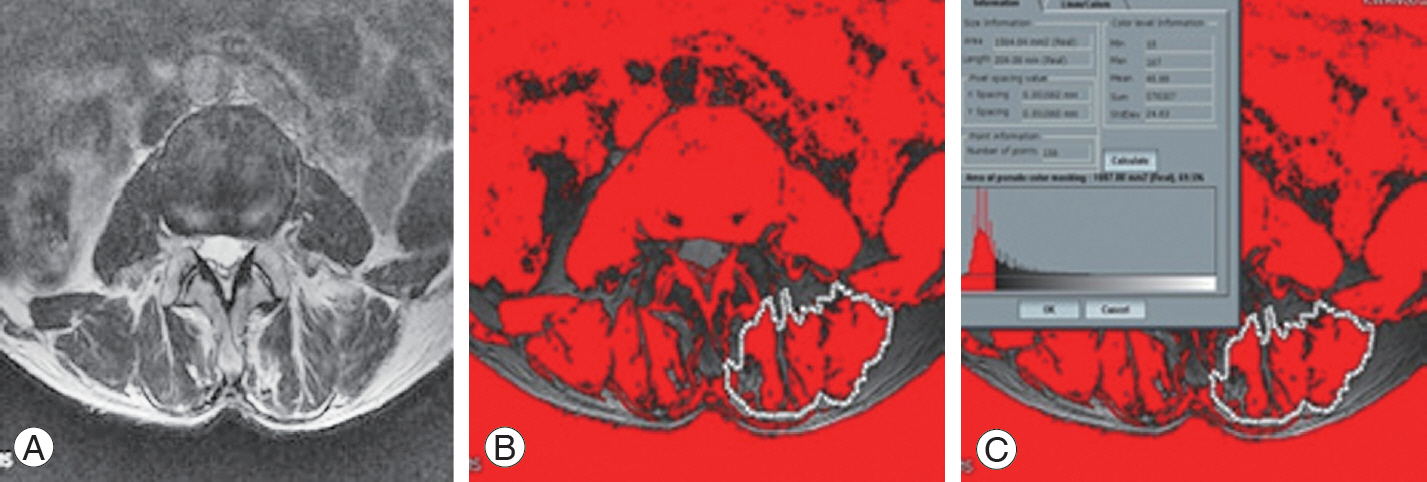
Fig. 2.
ROC curve analysis between fat degeneration of lumbar extensor muscle and osteoporotic vertebral fractures showed that the cut-off
value of fat degeneration was 31.90% (sensitivity=0.67, specificity=0.66). ROC, receiver operating characteristic.
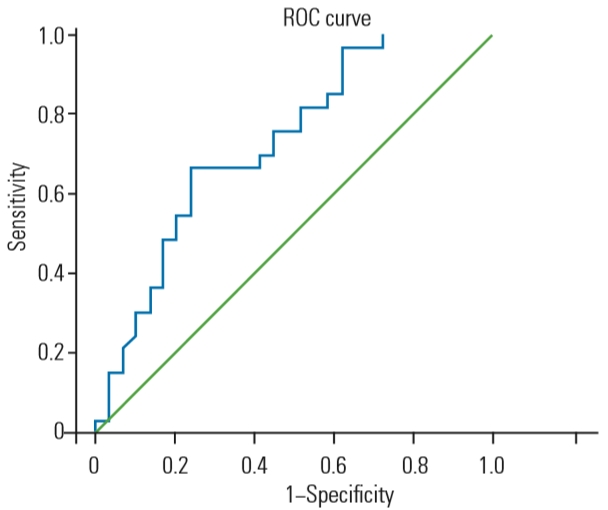
Table 1.
Comparison of basic data between two groups
| Variable | Group 1 (n=33) | Group 2 (n=29) | p-value |
|---|---|---|---|
| Bone mineral density | -3.36±1.76 | -1.81±1.30 | 0.01* |
| Gait speed test (m/sec) | 0.42±0.18 | 0.60±0.20 | 0.01* |
| Handgrip strength (kg) | 11.93±4.39 | 15.62±5.41 | 0.01* |
| Skeletal muscle index (kg/m2) | 5.49±1.08 | 6.32±0.77 | 0.01* |
| Paralumbar muscle mass (mm2) | 979.07±358.83 | 1,221.75±281.44 | 0.01* |
| Fat degeneration of lumbar extensor muscle (%) | 38.29±12.72 | 28.96±11.02 | 0.01* |
References
1. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101.


2. Cesari M, Fielding RA, Pahor M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:181–90.



3. Bahat G, Tufan A, Tufan F, et al. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 2016;35:1557–63.


4. Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc 2013;61:974–80.


5. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006.


6. Holt DQ, Strauss BJ, Lau KK, Moore GT. Body composition analysis using abdominal scans from routine clinical care in patients with Crohn’s disease. Scand J Gastroenterol 2016;51:842–7.


7. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31.



8. Yoo JI, Choi H, Ha YC. Mean hand grip strength and cut-off value for sarcopenia in Korean adults using KNHANES VI. J Korean Med Sci 2017;32:868–72.



9. Hogrel JY. Grip strength measured by high precision dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet Disord 2015;16:139.




10. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31.



11. Munoz-Mendoza CL, Cabanero-Martínez MJ, Millan-Calenti JC, Cabrero-Garcia J, Lopez-Sanchez R, Maseda-Rodriguez A. Reliability of 4-m and 6-m walking speed tests in elderly people with cognitive impairment. Arch Gerontol Geriatr 2011;52:e67–70.


12. Kim KE, Jang SN, Lim S, et al. Relationship between muscle mass and physical performance: is it the same in older adults with weak muscle strength? Age Ageing 2012;41:799–803.



13. Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine (Phila Pa 1976) 2008;33:318–25.


14. Abbas J, Slon V, May H, Peled N, Hershkovitz I, Hamoud K. Paraspinal muscles density: a marker for degenerative lumbar spinal stenosis? BMC Musculoskelet Disord 2016;17:422.




15. Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 2007;5:2.




16. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults: current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–56.



17. Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK, ILAS Research Group. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc 2013;14:528.

18. Beaudart C, McCloskey E, Bruyere O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016;16:170.




19. Feyerabend AJ, Lear JL. Regional variations in bone mineral density as assessed with dual-energy photon absorptiometry and dual X-ray absorptiometry. Radiology 1993;186:467–9.


20. Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ 3rd. The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int 2010;21:41–52.



21. Briggs AM, Greig AM, Wark JD, Fazzalari NL, Bennell KL. A review of anatomical and mechanical factors affecting vertebral body integrity. Int J Med Sci 2004;1:170–80.










