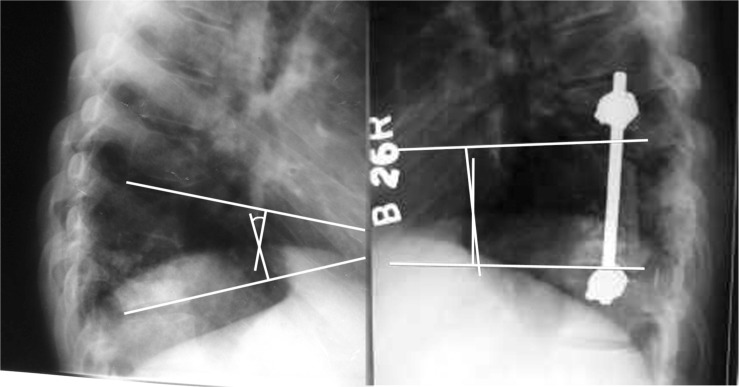
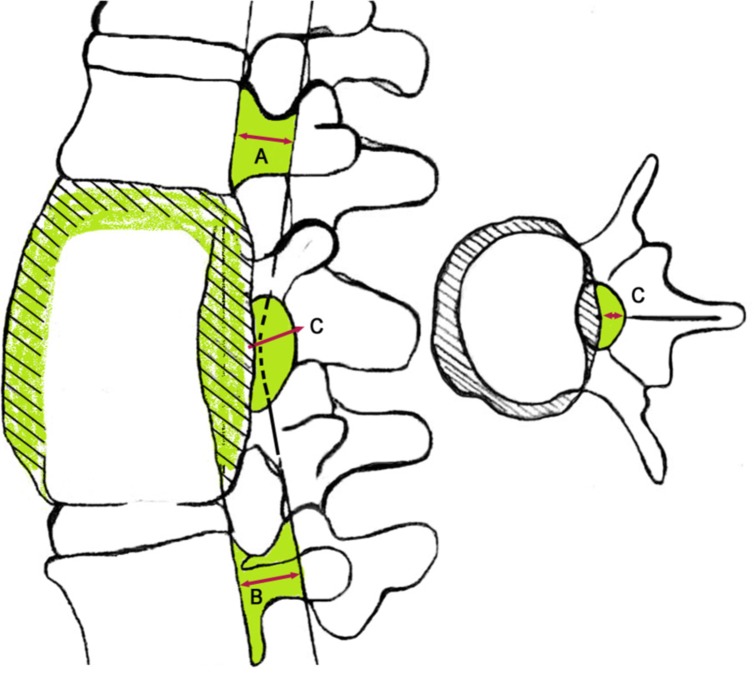
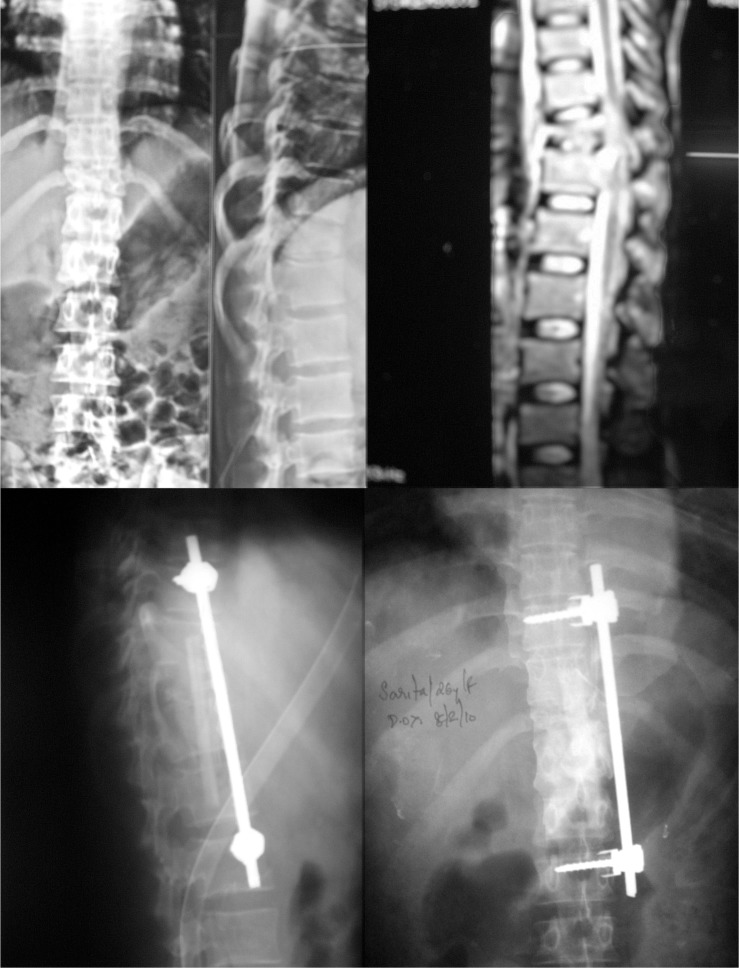
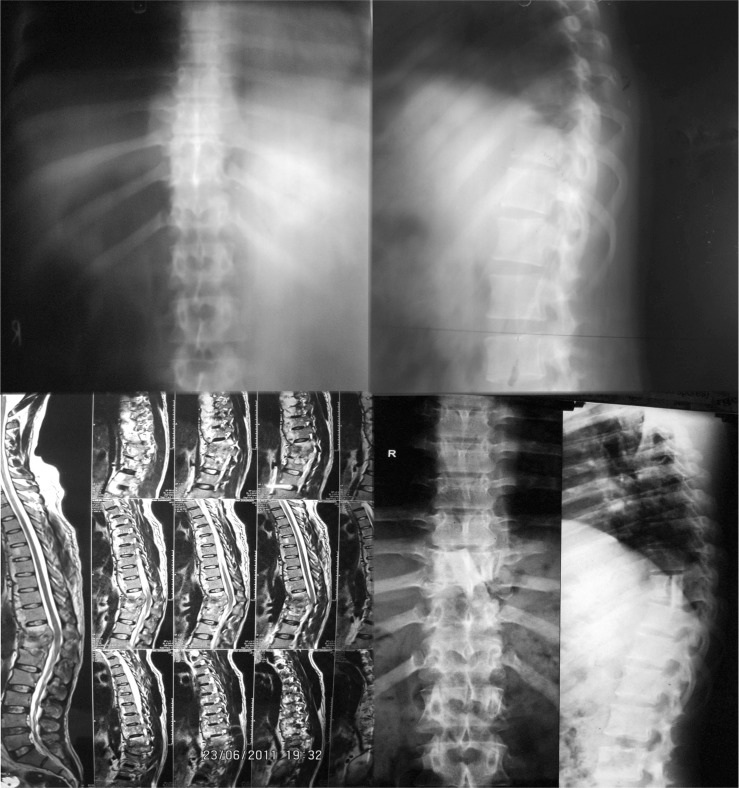
1. Ozdemir HM, Us AK, Oğün T. The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine (Phila Pa 1976) 2003 28:474–479. PMID:
12616160.


2. Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. J Bone Joint Surg Am 1987 69:503–509. PMID:
3571308.


3. Guven O. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop 1996 20:271PMID:
8872553.

4. Rezai AR, Lee M, Cooper PR, Errico TJ, Koslow M. Modern management of spinal tuberculosis. Neurosurgery 1995 36:87–97. PMID:
7708173.


5. Benli IT, Alanay A, Akalin S, et al. Comparison of anterior instrumentation systems and the results of minimum 5 years follow-up in the treatment of tuberculosis spondylitis. Kobe J Med Sci 2004 50:167–180. PMID:
16107774.

6. Chen CL, Chou CW, Su WW, Cheng CY, Yu CT. Dislodged upper thoracic cage in the gastrointestinal tract: a case report and literature reviews. Spine (Phila Pa 1976) 2008 33:E802–E806. PMID:
18827687.


7. Korovessis P, Petsinis G, Koureas G, Iliopoulos P, Zacharatos S. Anterior surgery with insertion of titanium mesh cage and posterior instrumented fusion performed sequentially on the same day under one anesthesia for septic spondylitis of thoracolumbar spine: is the use of titanium mesh cages safe? Spine (Phila Pa 1976) 2006 31:1014–1019. PMID:
16641778.


8. Oguz E, Sehirlioglu A, Altinmakas M, et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop 2008 32:127–133. PMID:
17206497.


9. Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 1969 7:179–192. PMID:
5360915.


10. Jain AK, Aggarwal A, Dhammi IK, Aggarwal PK, Singh S. Extrapleural anterolateral decompression in tuberculosis of the dorsal spine. J Bone Joint Surg Br 2004 86:1027–1031. PMID:
15446532.


11. Slucky AV, Eismont FJ,Bridwell KH, Dewald RL,Spinal infections. editors. The textbook of spinal surgery. 1997.Philadelphia: Lippincott; p.2141–2183.
12. Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clinical and biologic study. Spine (Phila Pa 1976) 1993 18:1890–1894. PMID:
8235878.


13. Babhulkar SS, Tayade WB, Babhulkar SK. Atypical spinal tuberculosis. J Bone Joint Surg Br 1984 66:239–242. PMID:
6707060.


14. Arthornthurasook A, Chongpieboonpatana A. Spinal tuberculosis with posterior element involvement. Spine (Phila Pa 1976) 1990 15:191–194. PMID:
2353255.


15. Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop 1995 19:327–331. PMID:
8567145.


16. Aksoy MC, Acaroglu RE, Tokgozoglu AM, Ozdemir N, Surat A. Retrospective evaluation of treatment methods in tuberculosis spondylitis. Hacettepe J Orthop Surg 1995 5:207–209.
17. Hodgson AR, Stock FE. Anterior spinal fusion. A preliminary communication on the radical treatment of Pott's disease and Pott's paraplegia. 1956. Clin Orthop Relat Res 1994 (300): 16–23. PMID:
8131329.

18. Upadhyay SS, Saji MJ, Sell P, Yau AC. The effect of age on the change in deformity after radical resection and anterior arthrodesis for tuberculosis of the spine. J Bone Joint Surg Am 1994 76:701–708. PMID:
8175818.


19. Yilmaz C, Selek HY, Gürkan I, Erdemli B, Korkusuz Z. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg Am 1999 81:1261–1267. PMID:
10505522.


20. Benli IT, Acaroğlu E, Akalin S, Kiş M, Duman E, Un A. Anterior radical debridement and anterior instrumentation in tuberculosis spondylitis. Eur Spine J 2003 12:224–234. PMID:
12709862.


21. Hassan MG. Anterior plating for lower cervical spine tuberculosis. Int Orthop 2003 27:73–77. PMID:
12700928.


22. Hodgson AR, Stock FE, Fang HS, Ong GB. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott's disease of the spine. Br J Surg 1960 48:172–178. PMID:
13714863.


23. Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treated by anterior arthrodesis. J Bone Joint Surg Am 1989 71:1314–1323. PMID:
2793883.


24. Loembe PM. Medical-surgical treatment of progressive tuberculous (Pott's) paraplegia in Gabon. Paraplegia 1995 33:579–584. PMID:
8848312.



25. Schulitz KP, Kothe R, Leong JC, Wehling P. Growth changes of solidly fused kyphotic bloc after surgery for tuberculosis. Comparison of four procedures. Spine (Phila Pa 1976) 1997 22:1150–1155. PMID:
9160475.


26. A 15-year assessment of controlled trials of the management of tuberculosis of the spine in Korea and Hong Kong. Thirteenth report of the medical research council working party on tuberculosis of the spine. J Bone Joint Surg Br 1998 80:456–462. PMID:
9619936.


27. Benli IT, Aydın E, Kis M, Akalın S, Tuzuner M, Baz AB. The results of anterior instrumentation in vertebral tuberculosis. J Turkish Spine Surg 1996 7:98–101.
28. Benli IT, Kiş M, Akalin S, Citak M, Kanevetçi S, Duman E. The results of anterior radical debridement and anterior instrumentation in Pott's disease and comparison with other surgical techniques. Kobe J Med Sci 2000 46:39–68. PMID:
11193503.

29. Jain AK. Tuberculosis of the spine: a fresh look at an old disease. J Bone Joint Surg Br 2010 92:905–913. PMID:
20595106.


30. Jain AK, Dhammi IK, Jain S, Mishra P. Kyphosis in spinal tuberculosis: prevention and correction. Indian J Orthop 2010 44:127–136. PMID:
20418999.



31. Rajasekaran S. The problem of deformity in spinal tuberculosis. Clin Orthop Relat Res 2002 (398): 85–92. PMID:
11964635.


32. Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs. A review of one hundred forty-six patients. Spine (Phila Pa 1976) 1984 9:667–671. PMID:
6505833.


33. Tuli SM. Tuberculosis of the skeletal system: bones, joints, spine and bursal sheaths. 1991.New Delhi: Jaypee Brothers Medical;